Abstract
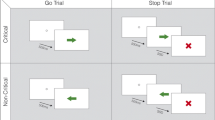
Recent imaging studies in healthy controls with a conditional stop signal reaction time (RT) task have implicated the subthalamic nucleus (STN) in response inhibition and the pre-supplementary motor area (pre-SMA) in conflict resolution. Parkinson’s disease (PD) is characterized by striatal dopamine deficiency and overactivity of the STN and underactivation of the pre-SMA during movement. We used the conditional stop signal RT task to investigate whether PD produced similar or dissociable effects on response initiation, response inhibition and response initiation under conflict. In addition, we also examined inhibition of prepotent responses on three cognitive tasks: the Stroop, random number generation and Hayling sentence completion. PD patients were impaired on the conditional stop signal reaction time task, with response initiation both in situations with or without conflict and response inhibition all being significantly delayed, and had significantly greater difficulty in suppressing prepotent or habitual responses on the Stroop, Hayling and random number generation tasks relative to controls. These results demonstrate the existence of a generalized inhibitory deficit in PD, which suggest that PD is a disorder of inhibition as well as activation and that in situations of conflict, executive control over responses is compromised.





Similar content being viewed by others
References
Alexander GE, DeLong MR, Strick PL (1986) Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 9:357–381
Aron AR, Behrens TE, Smith S, Frank MJ, Poldrack RA (2007) Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. J Neurosci 27(14):3743–3752
Baglio F, Blasi V, Falini A, et al. (2009) Functional brain changes in early Parkinson’s disease during motor response and motor inhibition. Neurobiol Aging. doi:10.1016/j.neurobiolaging.2008.12.009
Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J (1961) An inventory for measuring depression. Arch Gen Psychiatry 4:561–567
Beste C, Willemssen R, Saft C, Falkenstein M (2009) Response inhibition subprocesses and dopaminergic pathways: basal ganglia disease effects. Neuropsychologia. doi:10.1016/j.neuropsychologia.2009.09.023
Bokura H, Yamaguchi S, Kobayashi S (2005) Event-related potentials for response inhibition in Parkinson’s disease. Neuropsychologia 43:967–975. doi:10.1016/j.neuropsychologia.2004.08.010
Bouquet CA, Bonnaud V, Gil R (2003) Investigation of supervisory attentional system functions in patients with Parkinson’s disease using the Hayling task. J Clin Exp Neuropsychol 25:751–760
Brown RG, Marsden CD (1988) Internal versus external cues and the control of attention in Parkinson’s disease. Brain 111(Pt 2):323–345
Brown RG, Marsden CD (1991) Dual task performance and processing resources in normal subjects and patients with Parkinson’s disease. Brain 114(Pt 1A):215–231
Brown RG, Soliveri P, Jahanshahi M (1998) Executive processes in Parkinson’s disease-random number generation and response suppression. Neuropsychologia 36:1355–1362
Burgess PW, Shallice T (1997) The Hayling and Brixton tests. Thames Valley Test Company Limited, Bury St Edmunds
Cagigas XE, Filoteo JV, Stricker JL, Rilling LM, Friedrich FJ (2007) Flanker compatibility effects in patients with Parkinson’s disease: impact of target onset delay and trial-by-trial stimulus variation. Brain Cogn 63:247–259. doi:10.1016/j.bandc.2006.09.002
Chamberlain SR, Muller U, Blackwell AD, Clark L, Robbins TW, Sahakian BJ (2006) Neurochemical modulation of response inhibition and probabilistic learning in humans. Science 311:861–863. doi:10.1126/science.1121218
Chamberlain SR, Del Campo N, Dowson J, Muller U, Clark L, Robbins TW, Sahakian BJ (2007) Atomoxetine improved response inhibition in adults with attention deficit/hyperactivity disorder. Biol Psychiatry 62:977–984. doi:10.1016/j.biopsych.2007.03.003
Chamberlain SR, Hampshire A, Muller U et al (2009) Atomoxetine modulates right inferior frontal activation during inhibitory control: a pharmacological functional magnetic resonance imaging study. Biol Psychiatry 65:550–555. doi:10.1016/j.biopsych.2008.10.014
Chan F, Armstrong IT, Pari G, Riopelle RJ, Munoz DP (2005) Deficits in saccadic eye-movement control in Parkinson’s disease. Neuropsychologia 43:784–796. doi:10.1016/j.neuropsychologia.2004.06.026
Chevalier G, Deniau JM (1990) Disinhibition as a basic process in the expression of striatal functions. Trends Neurosci 13:277–280
Cools R, Barker RA, Sahakian BJ, Robbins TW (2001) Enhanced or impaired cognitive function in Parkinson’s disease as a function of dopaminergic medication and task demands. Cereb Cortex 11:1136–1143
Cools R, Clark L, Owen AM, Robbins TW (2002) Defining the neural mechanisms of probabilistic reversal learning using event-related functional magnetic resonance imaging. J Neurosci 22:4563–4567
Cools R, Barker RA, Sahakian BJ, Robbins TW (2003) L-Dopa medication remediates cognitive inflexibility, but increases impulsivity in patients with Parkinson’s disease. Neuropsychologia 41:1431–1441
Cooper JA, Sagar HJ, Tidswell P, Jordan N (1994) Slowed central processing in simple and go/no-go reaction time tasks in Parkinson’s disease. Brain 117(Pt 3):517–529
Delis DC, Kaplan E, Kramer JH (2001) Delis–Kaplan executive function scale. The Psychological Corporation, San Antonio
DeLong MR (1990) Primate models of movement disorders of basal ganglia origin. Trends Neurosci 13:281–285
Dirnberger G, Frith CD, Jahanshahi M (2005) Executive dysfunction in Parkinson’s disease is associated with altered pallidal-frontal processing. Neuroimage 25:588–599. doi:10.1016/j.neuroimage.2004.11.023
Eagle DM, Baunez C (2010) Is there an inhibitory-response-control system in the rat? Evidence from anatomical and pharmacological studies of behavioral inhibition. Neurosci Biobehav Rev 34:50–72
Falkenstein M, Hielscher H, Dziobek I, Schwarzenau P, Hoormann J, Sunderman B, Hohnsbein J (2001) Action monitoring, error detection, and the basal ganglia: an ERP study. Neuroreport 12:157–161
Falkenstein M, Willemssen R, Hohnsbein J, Hielscher H (2006) Effects of stimulus–response compatibility in Parkinson’s disease: a psychophysiological analysis. J Neural Transm 113:1449–1462. doi:10.1007/s00702-005-0430-1
Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198
Frank MJ, Samanta J, Moustafa AA, Sherman SJ (2007) Hold your horses: impulsivity, deep brain stimulation, and medication in Parkinsonism. Science 318:1309–1312. doi:10.1126/science.1146157
Gauggel S, Rieger M, Feghoff TA (2004) Inhibition of ongoing responses in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry 75:539–544
Harnishfeger KK (1995) The development of cognitive inhibition: Theories, definitions, and research evidence. In: Dempster FN, Brainerd CJ (eds) Interference and inhibition in cognition. Academic Press, San Diego
Hayes AE, Davidson MC, Keele SW, Rafal RD (1998) Toward a functional analysis of the basal ganglia. J Cogn Neurosci 10:178–198
Hershey T, Revilla FJ, Wernle A, Gibson PS, Dowling JL, Perlmutter JS (2004) Stimulation of STN impairs aspects of cognitive control in PD. Neurology 62:1110–1114
Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55:181–184
Isoda M, Hikosaka O (2007) Switching from automatic to controlled action by monkey medial frontal cortex. Nat Neurosci 10:240–248. doi:10.1038/nn1830
Isoda M, Hikosaka O (2008) Role for subthalamic nucleus neurons in switching from automatic to controlled eye movement. J Neurosci 28:7209–7218. doi:10.1523/JNEUROSCI.0487-08.2008
Jahanshahi M, Jenkins IH, Brown RG, Marsden CD, Passingham RE, Brooks DJ (1995) Self-initiated versus externally triggered movements. I. An investigation using measurement of regional cerebral blood flow with PET and movement-related potentials in normal and Parkinson’s disease subjects. Brain 118(Pt 4):913–933
Jahanshahi M, Profice P, Brown RG, Ridding MC, Dirnberger G, Rothwell JC (1998) The effects of transcranial magnetic stimulation over the dorsolateral prefrontal cortex on suppression of habitual counting during random number generation. Brain 121:1533–1544
Jahanshahi M, Ardouin CM, Brown RG et al (2000) The impact of deep brain stimulation on executive function in Parkinson’s disease. Brain 123(Pt 6):1142–1154
Kuhn AA, Tsui A, Aziz T et al (2009) Pathological synchronisation in the subthalamic nucleus of patients with Parkinson’s disease relates to both bradykinesia and rigidity. Exp Neurol 215:380–387. doi:10.1016/j.expneurol.2008.11.008
Lee SS, Wild K, Hollnagel C, Grafman J (1999) Selective visual attention in patients with frontal lobe lesions or Parkinson’s disease. Neuropsychologia 37:595–604
Logan GD, Cowan WB (1984) On the ability to inhibit thought and action: a theory of an act of control. Psychol Rev 91:295–327
Middleton FA, Strick PL (2000) Basal ganglia output and cognition: evidence from anatomical, behavioral, and clinical studies. Brain Cogn 42:183–200
Nigg JT (2000) On inhibition/disinhibition in developmental psychopathology: views from cognitive and personality psychology and a working inhibition taxonomy. Psychol Bull 126:220–246
Owen AM, Roberts AC, Polkey CE, Sahakian BJ, Robbins TW (1991) Extra-dimensional versus intra-dimensional set shifting performance following frontal lobe excisions, temporal lobe excisions or amygdalo-hippocampectomy in man. Neuropsychologia 29:993–1006
Playford ED, Jenkins IH, Passingham RE, Nutt J, Frackowiak RS, Brooks DJ (1992) Impaired mesial frontal and putamen activation in Parkinson’s disease: a positron emission tomography study. Ann Neurol 32:151–161. doi:10.1002/ana.410320206
Praamstra P, Plat FM (2001) Failed suppression of direct visuomotor activation in Parkinson’s disease. J Cogn Neurosci 13:31–43
Praamstra P, Stegeman DF, Cools AR, Horstink MW (1998) Reliance on external cues for movement initiation in Parkinson’s disease. Evidence from movement-related potentials. Brain 121(Pt 1):167–177
Ray NJ, Jenkinson N, Brittain J et al (2009) The role of the subthalamic nucleus in response inhibition: evidence from deep brain stimulation for Parkinson’s disease. Neuropsychologia 47:2828–2834. doi:10.1016/j.neuropsychologia.2009.06.011
Redgrave P, Prescott TJ, Gurney K (1999) The basal ganglia: a vertebrate solution to the selection problem? Neuroscience 89:1009–1023
Rieger M, Gauggel S (1999) Inhibitory after-effects in the stop-signal paradigm. Br J Psychol 90:509–518
Rieger M, Gauggel S, Burmeister K (2003) Inhibition of ongoing responses following frontal, nonfrontal, and basal ganglia lesions. Neuropsychology 17:272–282
Rivaud-Pechoux S, Vidailhet M, Brandel JP, Gaymard B (2007) Mixing pro- and antisaccades in patients with parkinsonian syndromes. Brain 130:256–264. doi:10.1093/brain/awl315
Schroeder U, Kuehler A, Haslinger B et al (2002) Subthalamic nucleus stimulation affects striato-anterior cingulate cortex circuit in a response conflict task: a PET study. Brain 125:1995–2004
Seiss E, Praamstra P (2004) The basal ganglia and inhibitory mechanisms in response selection: evidence from subliminal priming of motor responses in Parkinson’s disease. Brain 127:330–339. doi:10.1093/brain/awh043
Seiss E, Praamstra P (2006) Time-course of masked response priming and inhibition in Parkinson’s disease. Neuropsychologia 44:869–875
Spatt J, Goldenberg G (1993) Components of random generation by normal subjects and patients with dysexecutive syndrome. Brain Cogn 23:231–242
Stroop JR (1935) Studies of interference in serial verbal reactions. J Exp Psychol 18:643–662
Thobois S, Hotton GR, Pinto S, Wilkinson L, Limousin-Dowsey P, Brooks DJ, Jahanshahi M (2007) STN stimulation alters pallidal-frontal coupling during response selection under competition. J Cereb Blood Flow Metab 27:1173–1184
van den Wildenberg WP, van Boxtel GJ, van der Molen MW, Bosch DA, Speelman JD, Brunia CH (2006) Stimulation of the subthalamic region facilitates the selection and inhibition of motor responses in Parkinson’s disease. J Cogn Neurosci 18:626–636
Verbruggen F, Logan GD (2009a) Automaticity of cognitive control: goal priming in response-inhibition paradigms. J Exp Psychol Learn Mem Cogn 35:1381–1388. doi:10.1037/a0016645
Verbruggen F, Logan GD (2009b) Proactive adjustments of response strategies in the stop-signal paradigm. J Exp Psychol Hum Percept Perform 35:835–854. doi:10.1037/a0012726
Vila M, Levy R, Herrero MT et al (1997) Consequences of nigrostriatal denervation on the functioning of the basal ganglia in human and nonhuman primates: an in situ hybridization study of cytochrome oxidase subunit I mRNA. J Neurosci 17:765–773
Voon V, Fernagut PO, Wickens J et al (2009) Chronic dopaminergic stimulation in Parkinson’s disease: from dyskinesias to impulse control disorders. Lancet Neurol 8:1140–1149. doi:10.1016/S1474-4422(09)70287-X
Williams-Gray CH, Foltynie T, Brayne CE, Robbins TW, Barker RA (2007) Evolution of cognitive dysfunction in an incident Parkinson’s disease cohort. Brain 130:1787–1798. doi:10.1093/brain/awm111
Witt K, Pulkowski U, Herzog J, Lorenz D, Hamel W, Deuschl G, Krack P (2004) Deep brain stimulation of the subthalamic nucleus improves cognitive flexibility but impairs response inhibition in Parkinson disease. Arch Neurol 61:697–700. doi:10.1001/archneur.61.5.697
Wylie SA, Stout JC, Bashore TR (2005) Activation of conflicting responses in Parkinson’s disease: evidence for degrading and facilitating effects on response time. Neuropsychologia 43:1033–1043
Wylie SA, van den Wildenberg WP, Ridderinkhof KR, Bashore TR, Powell VD, Manning CA, Wooten GF (2009a) The effect of Parkinson’s disease on interference control during action selection. Neuropsychologia 47:145–157. doi:10.1016/j.neuropsychologia.2008.08.001
Wylie SA, van den Wildenberg WP, Ridderinkhof KR, Bashore TR, Powell VD, Manning CA, Wooten GF (2009b) The effect of speed-accuracy strategy on response interference control in Parkinson’s disease. Neuropsychologia 47:1844–1853. doi:10.1016/j.neuropsychologia.2009.02.025
Acknowledgments
We are grateful to all the participants. This work was supported by a PhD studentship from Fundación Caja Madrid (IO), a Career Development Fellowship from the Parkinson’s disease Society (LW) and a Royal Society Travelling Fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Obeso, I., Wilkinson, L., Casabona, E. et al. Deficits in inhibitory control and conflict resolution on cognitive and motor tasks in Parkinson’s disease. Exp Brain Res 212, 371–384 (2011). https://doi.org/10.1007/s00221-011-2736-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-011-2736-6