Abstract
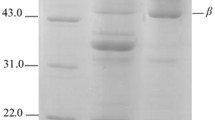
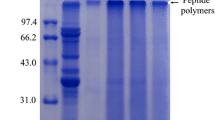
Soybean is a high-quality plant protein resource and also a major food allergen. Glycation is widely used to modify protein allergens. In the current report, the influences of different saccharides on soybean protein structure and antigenicity through glycation were investigated. Soybean protein isolate (SPI) and saccharides (glucose, galactose, maltose, lactose, and dextran), at 1:1 weight ratio, were dry-heated at 60 °C and 79 % relative humidity for different times. The content of free amino group in glycated products was decreased by trinitrobenzene sulfonic acid method. In addition, high-molecular aggregates were generated in the glycated SPI, indicating that glycation reaction occurred in SPI–saccharide conjugates. Moreover, the structure of SPI in conjugates changed with exposure to aromatic side chains. Of all the SPI–saccharide conjugates, with time increased from 0 to 72 h, the antigenicity inhibition rate of β-conglycinin in SPI–glucose complexes declined from 83.55 (0 h) to 29.80 % (48 h), suggesting that introducing saccharides in SPI is an effective method to reduce the antigenicity of β-conglycinin.





Similar content being viewed by others
References
Fritsche R (2003) Role of technology in dairy allergy. Aust J Dairy Technol 58:89–91
Tryphonas H, Arvanitakis G, Vavasour E, Bondy G (2003) Animal models to detect allergenicity to foods and genetically modified products: workshop summary. Environ Health Perspect 111:221
Shannon-Wilson BS, Blaschek K, Mejia EG (2005) Allergenic proteins in soybean: processing and reduction of P34 allergenicity. Nutr Rev 63:47–58
Gomaa A, Boye JI (2013) Impact of thermal processing time and cookie size on the detection of casein, egg, gluten and soy allergens in food. Food Res Int 52:483–489
Cucu T, Platteau C, Taverniers I, Devreese B, De Loose M, De Meulenaer B (2013) Effect of partial hydrolysis on the hazelnut and soybean protein detectability by ELISA. Food Control 30:497–503
Song YS, Frias J, Martinez-Villaluenga C, Vidal-Valdeverde C, De Mejia EG (2008) Immunoreactivity reduction of soybean meal by fermentation, effect on amino acid composition and antigenicity of commercial soy products. Food Chem 108:571–581
Usui M, Tamura H, Nakamura K, Ogawa T, Muroshita M, Azakami H, Kato A (2004) Enhanced bactericidal action and masking of allergen structure of soy protein by attachment of chitosan through Maillard-type protein–polysaccharide conjugation. Food/Nahrung 48:69–72
Holzhauser T, Wackermann O, Ballmer-Weber BK, Bindslev-Jensen C, Scibilia J, Perono-Garoffo L, Utsumi S, Poulsen LK, Vieths S (2008) Soybean (Glycine max) allergy in Europe: gly m 5 (β-conglycinin) and Gly m 6 (glycinin) are potential diagnostic markers for severe allergic reactions to soy. J Allergy Clin Immunol 123:453–458
Maruyama N, Katsube T, Wada Y, Oh MH, Barba De La Rosa AP, Okuda E, Utsumi S (1998) The roles of the N-linked glycans and extension regions of soybean β-conglycinin in folding, assembly and structural features. Eur J Biochem 258:854–862
Prak K, Nakatani K, Katsube-Tanaka T, Adachi M, Maruyama N, Utsumi S (2005) Structure–function relationships of soybean proglycinins at subunit levels. J Agric Food Chem 53:3650–3657
Bu G, Luo Y, Chen F, Liu K, Zhu T (2013) Milk processing as a tool to reduce cow’s milk allergenicity: a mini-review. Dairy Sci Technol 93:211–223
Liu Y, Zhao G, Zhao M, Ren J, Yang B (2012) Improvement of functional properties of peanut protein isolate by conjugation with dextran through Maillard reaction. Food Chem 131:901–906
Hattori M, Miyakawa S, Ohama Y, Kawamura H, Yoshida T, To-o K, Takahashi K (2004) Reduced immunogenicity of β-lactoglobulin by conjugation with acidic oligosaccharides. J Agric Food Chem 52:4546–4553
Hiller B, Lorenzen PC (2010) Functional properties of milk proteins as affected by Maillard reaction induced oligomerisation. Food Res Int 43:1155–1166
Xue F, Li C, Zhu X, Wang L, Pan S (2013) Comparative studies on the physicochemical properties of soy protein isolate–maltodextrin and soy protein isolate-gum acacia conjugate prepared through Maillard reaction. Food Res Int 51:490–495
Van de Lagemaat J, Manuel Silván J, Javier Moreno F, Olano A, Dolores del Castillo M (2007) In vitro glycation and antigenicity of soy proteins. Food Res Int 40:153–160
Bielikowicz K, Kostyra H, Kostyra E, Teodorowicz M, Rigby N, Wojtacha P (2012) The influence of non-enzymatic glycosylation on physicochemical and biological properties of pea globulin 7S. Food Res Int 48:831–838
Zhang X, Qi J, Li K, Yin SW, Wang JM, Zhu JH, Yang XQ (2012) Characterization of soy β-conglycinin–dextran conjugate prepared by Maillard reaction in crowded liquid system. Food Res Int 49:648–654
Kasran M, Cui SW, Goff HD (2013) Covalent attachment of fenugreek gum to soy whey protein isolate through natural Maillard reaction for improved emulsion stability. Food Hydrocoll 30:552–558
Diftis N, Kiosseoglou V (2006) Physicochemical properties of dry-heated soy protein isolate–dextran mixtures. Food Chem 96:228–233
Nissen JN (1979) Determination of the degress of hydrolysis of food protein hydrolysates by trinitribenzenesulfonic acid. J Agric Food Chem 27:1256–1263
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacterophage T4. Nature 227:680–685
Tong P, Gao J, Chen H, Li X, Zhang Y, Jian S, Liu F (2012) Effect of heat treatment on the potential allergenicity and conformational structure of egg allergen ovotransferrin. Food Chem 131:603–610
Zhao X, Chen F, Xue W, Lee L (2008) FTIR spectra studies on the secondary structures of 7S and 11S globulins from soybean proteins using AOT reverse micellar exteaction. Food Hydrocoll 22:568–575
Bu G, Luo Y, Lu J, Zhang Y (2010) Reduced antigenicity of β-lactoglobulin by conjugation with glucose through controlled Maillard reaction conditions. Food Agric Immunol 21:143–156
Zhang M, Zheng J, Ge K, Zhang H, Fang B, Jiang L, Ren F (2014) Glycation of α-lactalbumin with different size saccharides: effect on protein structure and antigenicity. Int Dairy J 34:220–228
Jiang SJ, Zhao XH (2010) Transglutaminase-induced cross-linking and glucosamineconjugation in soybean protein isolates and its impacts on some functional properties of the products. Eur Food Res Technol 231:679–689
Taheri-Kafrani A, Gaudin JC, Rabesona H, Nioi C, Agarwal D, Drouet M, Haertle T (2009) Effects of heating and glycation of β-lactoglobulin on its recognition by IgE of sera from cow milk allergy patients. J Agric Food Chem 57:4974–4982
Bu G, Luo Y, Zheng Z, Zheng H (2009) Effect of heat treatment on the antigenicity of bovine a-lactalbumin and β-lactoglobulin in whey protein isolate. Food Agric Immunol 20:195–206
Shriver SK, Yang WW (2011) Thermal and nonthermal methods for food allergen control. Food Eng Rev 3:26–43
Pallarès I, Vendrell J, Avilès FX, Ventura S (2004) Amyloid fibril formation by a partially structured intermediate state of α-chymotrypsin. J Mol Biol 342:321–331
Chen X, Ru Y, Chen F, Wang X, Zhao X, Ao Q (2013) FTIR spectroscopic characterization of soy proteins obtained through AOT reverse micelles. Food Hydrocoll 31:435–437
Hou HJ, Chang KC (2004) Structural characteristics of purified β-conglycinin from soybean stored under four conditions. J Agric Food Chem 52:7931–7937
Kleber N, Maier S, Hinrichs J (2007) Antigenic response of bovine β-lactoglobulin influenced by ultra-high pressure treatment and temperature. Innov Food Sci Emerg Technol 8:39–45
Zhao YN, **n Y, Jiang ZM (2010) Whey protein glycosylation reaction properties and oxidation resistance. China Dairy Industry 8:18–21
Li Y, Lu F, Luo CR (2009) Functional properties of the Maillard reaction products of rice protein with sugar. Food Chem 117:69–74
Acknowledgments
This work was supported financially by the National Natural Science Foundation of China (31201293 and 31171790), the National High Technology Research and Development Program of China (863 Program) (2013AA102208-5) and Foundation of Henan Educational Committee (14B550013).
Conflict of Interest
None.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bu, G., Zhu, T., Chen, F. et al. Effects of saccharide on the structure and antigenicity of β-conglycinin in soybean protein isolate by glycation. Eur Food Res Technol 240, 285–293 (2015). https://doi.org/10.1007/s00217-014-2326-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-014-2326-5