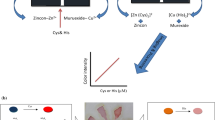
Abstract
This manuscript reports on a simple paper-based bienzymatic colorimetric assay for sarcosine as an important urinary biomarker of prostate cancer. All required assay reagents are pre-deposited on hydrophilic filter paper spots surrounded by a hydrophobic barrier. Sarcosine in the sample solution is selectively oxidized in the presence of sarcosine oxidase (SOx), resulting in the formation of hydrogen peroxide, which is subsequently detected through the horseradish peroxidase (HRP)–catalyzed conversion of the colorless indicator 3,3’,5,5’-tetramethylbenzidine (TMB) into its blue-colored oxidation product. By the modification of the paper with positively charged poly(allylamine hydrochloride) (PAH), a linear response to sarcosine between 0 and 10 μM and a significant lowering of the limit of detection (LOD) (0.6 μM) compared to the unmodified paper substrate (12.6 μM) has been achieved. The improvement of the LOD was attributed to the fact that the presence of the polymer limits the enzyme-driven colorimetric reaction to the surface of the paper substrate, resulting in stronger color development. In experiments in artificial urine matrix, the bicarbonate anion was identified as an inhibitor of the colorimetric reaction. This inhibition was successfully eliminated through on-device sample pH adjustments with pH-buffer components pre-deposited onto assay devices. The LOD for sarcosine achieved in artificial urine matrix (2.5 μM) is below the 5 μM threshold value for this urinary biomarker required for diagnostic purposes. Finally, good selectivity over all 20 natural amino acids and satisfactory long-term storage stability of reagent-modified paper substrates at − 20 °C for a period of 50 days were confirmed.
Graphical abstract











Similar content being viewed by others
References
Couzin J. Metabolite in urine may point to high-risk prostate cancer. Science. 2009;323:865.
Katz A. Ce: early localized prostate cancer. Am J Nurs. 2015;115:34–44.
Merriel SWD, Funston G, Hamilton W. Prostate cancer in primary care. Adv Ther. 2018;35:1285–94.
Schiffer E. Biomarkers for prostate cancer. World J Urol. 2007;25:557–62.
Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, et al. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009;457:910–4.
Jiang Y, Cheng X, Wang C, Ma Y. Quantitative determination of sarcosine and related compounds in urinary samples by liquid chromatography with tandem mass spectrometry. Anal Chem. 2010;82:9022–7.
Narwal V, Kumar P, Joon P, Pundir CS. Fabrication of an amperometric sarcosine biosensor based on sarcosine oxidase/chitosan/CuNPs/c-MWCNT/au electrode for detection of prostate cancer. Enzym Microb Technol. 2018;113:44–51.
Liu T, Fu B, Chen J, Li K. An electrochemical sarcosine sensor based on biomimetic recognition. Microchim Acta. 2019;186:136.
Lan J, Xu W, Wan Q, Zhang X, Lin J, Chen J, et al. Colorimetric determination of sarcosine in urine samples of prostatic carcinoma by mimic enzyme palladium nanoparticles. Anal Chim Acta. 2014;825:63–8.
Uhlirova D, Stankova M, Docekalova M, Hosnedlova B, Kepinska M, Ruttkay-Nedecky B, et al. A rapid method for the detection of sarcosine using Spions/Au/CS/SOX/NPs for prostate cancer sensing. Int J Mol Sci. 2018;19:3722.
Burton C, Gamagedara S, Ma Y. A novel enzymatic technique for determination of sarcosine in urine samples. Anal Methods. 2012;4:141–6.
Heger Z, Cernei N, Krizkova S, Masarik M, Kopel P, Hodek P, et al. Paramagnetic nanoparticles as a platform for fret-based sarcosine picomolar detection. Sci Rep. 2015;5:8868.
Henderson CJ, Pumford E, Seevaratnam DJ, Daly R, Hall EAH. Gene to diagnostic: self immobilizing protein for silica microparticle biosensor, modelled with sarcosine oxidase. Biomaterials. 2019;193:58–70.
Jornet-Martínez N, Henderson CJ, Campíns-Falcó P, Daly R, Hall EAH. Towards sarcosine determination in urine for prostatic carcinoma detection. Sensors Actuators B Chem. 2019;287:380–9.
Martinez AW, Phillips ST, Butte MJ, Whitesides GM. Patterned paper as a platform for inexpensive, low-volume, portable bioassays. Angew Chem Int Ed. 2007;46:1318–20.
Yamada K, Shibata H, Suzuki K, Citterio D. Toward practical application of paper-based microfluidics for medical diagnostics: state-of-the-art and challenges. Lab Chip. 2017;17:1206–49.
Cate DM, Adkins JA, Mettakoonpitak J, Henry CS. Recent developments in paper-based microfluidic devices. Anal Chem. 2015;87:19–41.
Chen X, Chen J, Wang F, **ang X, Luo M, Ji X, et al. Determination of glucose and uric acid with bienzyme colorimetry on microfluidic paper-based analysis devices. Biosens Bioelectron. 2012;35:363–8.
Dungchai W, Chailapakul O, Henry CS. Electrochemical detection for paper-based microfluidics. Anal Chem. 2009;81:5821–6.
Feng Q-M, Pan J-B, Zhang H-R, Xu J-J, Chen H-Y. Disposable paper-based bipolar electrode for sensitive electrochemiluminescence detection of a cancer biomarker. Chem Commun. 2014;50:10949–51.
Chen X, Luo Y, Shi B, Liu X, Gao Z, Du Y, et al. Chemiluminescence diminishment on a paper-based analytical device: high throughput determination of β-agonists in swine hair. Anal Methods. 2014;6:9684–90.
Yamada K, Henares TG, Suzuki K, Citterio D. Distance-based tear lactoferrin assay on microfluidic paper device using interfacial interactions on surface-modified cellulose. ACS Appl Mater Interfaces. 2015;7:24864–75.
Martinez AW, Phillips ST, Carrilho E, Thomas SW, Sindi H, Whitesides GM. Simple telemedicine for develo** regions: camera phones and paper-based microfluidic devices for real-time, off-site diagnosis. Anal Chem. 2008;80:3699–707.
Morbioli GG, Mazzu-Nascimento T, Stockton AM, Carrilho E. Technical aspects and challenges of colorimetric detection with microfluidic paper-based analytical devices (μpads)- a review. Anal Chim Acta. 2017;970:1–22.
Mazzu-Nascimento T, Gomes Carneiro Leão PA, Catai JR, Morbioli GG, Carrilho E. Towards low-cost bioanalytical tools for sarcosine assays for cancer diagnostics. Anal Methods. 2016;8:7312–8.
de Tarso GP, Garcia Cardoso TM, Garcia CD, Carrilho E, Tomazelli Coltro WK. A handheld stam** process to fabricate microfluidic paper-based analytical devices with chemically modified surface for clinical assays. RSC Adv. 2014;4:37637–44.
Parween S, Asthana A. An affordable, rapid determination of total lipid profile using paper-based microfluidic device. Sensors Actuators B Chem. 2019;285:405–12.
Evans E, Moreira Gabriel EF, Benavidez TE, Tomazelli Coltro WK, Garcia CD. Modification of microfluidic paper-based devices with silica nanoparticles. Analyst. 2014;139:5560–7.
Wang S, Ge L, Song X, Yu J, Ge S, Huang J, et al. Paper-based chemiluminescence elisa: lab-on-paper based on chitosan modified paper device and wax-screen-printing. Biosens Bioelectron. 2012;31:212–8.
Gabriel EFM, Garcia PT, Cardoso TMG, Lopes FM, Martins FT, Coltro WKT. Highly sensitive colorimetric detection of glucose and uric acid in biological fluids using chitosan-modified paper microfluidic devices. Analyst. 2016;141:4749–56.
Kudo H, Yamada K, Watanabe D, Suzuki K, Citterio D. Paper-based analytical device for zinc ion quantification in water samples with power-free analyte concentration. Micromachines. 2017;8:127.
Ota R, Yamada K, Suzuki K, Citterio D. Quantitative evaluation of analyte transport on microfluidic paper-based analytical devices (μPADs). Analyst. 2018;143:643–53.
Liu S, Cao R, Wu J, Guan L, Li M, Liu J, et al. Directly writing barrier-free patterned biosensors and bioassays on paper for low-cost diagnostics. Sensors Actuators B Chem. 2019;285:529–35.
Alila S, Boufi S, Belgacem MN, Beneventi D. Adsorption of a cationic surfactant onto cellulosic fibers I. Surface charge effects. Langmuir. 2005;21:8106–13.
Carrilho E, Martinez AW, Whitesides GM. Understanding wax printing: a simple micropatterning process for paper-based microfluidics. Anal Chem. 2009;81:7091–5.
Lu Y, Shi W, Jiang L, Qin J, Lin B. Rapid prototy** of paper-based microfluidics with wax for low-cost, portable bioassay. Electrophoresis. 2009;30:1497–500.
Brooks T, Keevil CW. A simple artificial urine for the growth of urinary pathogens. Lett Appl Microbiol. 1997;24:203–6.
Porstmann T, Kiessig ST. Enzyme immunoassay techniques an overview. J Immunol Methods. 1992;150:5–21.
Harpaz D, Eltzov E, Ng TSE, Marks RS, Tok AIY. Enhanced colorimetric signal for accurate signal detection in paper-based biosensors. Diagnostics. 2020;10:28.
Zhang X, Yang Q, Lang Y, Jiang X, Wu P. Rationale of 3,3’,5,5’-tetramethylbenzidine as the chromogenic substrate in colorimetric analysis. Anal Chem. 2020;92:12400–6.
Currie LA. Limits for qualitative detection and quantitative determination. Application to radiochemistry. Anal Chem. 1968;40:586–93.
Armbruster DA, Pry T. Limit of blank, limit of detection and limit of quantitation. Clin Biochem Rev. 2008;29(Suppl 1):S49–52.
Suzuki M. Purification and some properties of sarcosine oxidase from Corynebacterium sp. U-96. J Biochem. 1981;89:599–607.
Katoh A, Maejima K, Hiruta Y, Citterio D. All-printed semiquantitative paper-based analytical devices relying on QR code array readout. Analyst. 2020;145:6071–8.
Zhou W, Sun J, Li X. Low-cost quantitative photothermal genetic detection of pathogens on a paper hybrid device using a thermometer. Anal Chem. 2020;92:14830–7.
Chattopadhyay K, Mazumdar S. Structural and conformational stability of horseradish peroxidase: effect of temperature and pH. Biochemistry. 2000;39:263–70.
Saito M, Itoh A, Suzuki H. Deuterium kinetic isotope effects in heterotetrameric sarcosine oxidase from Corynebacterium sp. U-96: the anionic form of the substrate in the enzyme–substrate complex is a reactive species. J Biochem. 2012;151:633–42.
Acknowledgements
We thank Mr. Kogi Kaizu of Keio University for his support with SEM image recording.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Published in the topical collection celebrating ABCs 20th Anniversary.
Supplementary information
ESM 1
(PDF 2732 kb)
Rights and permissions
About this article
Cite this article
Masumoto, M., Ohta, S., Nakagawa, M. et al. Colorimetric paper-based sarcosine assay with improved sensitivity. Anal Bioanal Chem 414, 691–701 (2022). https://doi.org/10.1007/s00216-021-03682-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-021-03682-0