Abstract
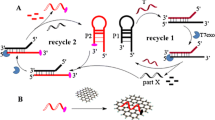
The reduced graphene oxide (rGO) could strongly adsorb and quench the fluorescence of dye-labeled single-stranded DNA (ssDNA); thus, it is widely applied in fluorescent sensors. However, these sensors may suffer from a limited sensitivity due to the low fluorescence recovery when adding the complementary DNA (cDNA) sequence. In this work, the powerful DNA branched junctions were constructed to improve the fluorescence recovery of FAM-labeled probe on rGO. In the presence of target Pb2+, the ribonucleotide (rA) in the substrate was cleaved specifically and the catalytic hairpin assembly of three metastable hairpins was further initiated, accompanied by the formation of DNA branched junctions. Then, the liberated Pb2+ could be recyclable. Impressively, the DNA branched junctions not only hybridize with the FAM-labeled probes with a high efficiency, but also are significantly undesirable for the rGO. Thus, a high fluorescence recovery of FAM-labeled probe on rGO was expected. The integration of the high fluorescence recovery and dual-cycle signal amplification endows the sensing strategy with a good performance for Pb2+ detection, including low detection limit (0.17 nM), good selectivity, and satisfactory practical applicability. The proposed DNA branched junctions offer a novel avenue to improve the fluorescence recovery of the dye-labeled probes on rGO for biological analysis.






Similar content being viewed by others
References
Vilela D, Parmar J, Zeng Y, Zhao Y, Sánchez S. Graphene-based microbots for toxic heavy metal removal and recovery from water. Nano Lett. 2016;16:2860–6.
Zhang YL, Chen WH, Dong XT, Fan H, Wang XH, Bian LJ. Simultaneous detection of trace toxic metal ions, Pb2+ and Ag+, in water and food using a novel single-labeled fluorescent oligonucleotide probe. Sensors Actuators B. 2018;261:58–65.
Nordberg GF, Fowler BA, Nordberg M, Frigerg L. Handbook on the toxicology of metals. 3rd ed. Burlington: Academic; 2007.
Yu Y, Hong Y, Gao P, Nazeeruddin MK. Glutathione modified gold nanoparticles for sensitive colorimetric detection of Pb2+ ions in rainwater polluted by leaking perovskite solar cells. Anal Chem. 2016;88:12316–22.
Liang P, Sang H. Determination of trace lead in biological and water samples with dispersive liquid–liquid microextraction preconcentration. Anal Biochem. 2008;380:21–5.
Peng YJ, Li Y, Li LL, Zhu JJ. A label-free aptasensor for ultrasensitive Pb2+ detection based on electrochemiluminescence resonance energy transfer between carbon nitride nanofibers and Ru(phen)32+. J Hazard Mater. 2018;359:121–8.
He Q, Miller EW, Wong AP, Chang CJ. A selective fluorescent sensor for detecting lead in living cells. J Am Chem Soc. 2006;128:9316–7.
Lu ZW, Lin XN, Zhang JJ, Dai WL, Liu BC, Mo GQ, et al. Ionic liquid/poly-l-cysteine composite deposited on flexible and hierarchical porous laser-engraved graphene electrode for high-performance electrochemical analysis of lead ion. Electrochim Acta. 2019;295:514–23.
Lu W, Qin X, Liu S, Chang G, Zhang Y, Luo Y, et al. Economical green synthesis of fluorescent carbon nanoparticles and their use as probes for sensitive and selective detection of mercury(II) ions. Anal Chem. 2012;84:5351–7.
Wang XZ, Jiang AW, Hou T, Li HY, Li F. Enzyme-free and label-free fluorescence aptasensing strategy for highly sensitive detection of protein based on target-triggered hybridization chain reaction amplification. Biosens Bioelectron. 2015;70:324–9.
Shomali Z, Kompany-Zareh M, Omidikia N. Fluorescence based investigation of temperature-dependent Pb2+-specific 8-17E DNAzyme catalytic sensor. J Fluoresc. 2019;29:335–42.
Zhou WH, Saran R, Liu JW. Metal sensing by DNA. Chem Rev. 2017;117(12):8272–325.
Chen M, Hassan M, Li HH, Chen QS. Fluorometric determination of lead(II) by using aptamer-functionalized upconversion nanoparticles and magnetite-modified gold nanoparticles. Microchim Acta. 2020;187:85.
Liu XL, Wang YZ, Song YJ. Visually multiplexed quantitation of heavy metal ions in water using volumetric bar-chart chip. Biosens Bioelectron. 2018;117:644–50.
Wen ZB, Liang WB, Zhuo Y, **ong CY, Zheng YN, Yuan R, et al. An efficient target-intermediate recycling amplification strategy for ultrasensitive fluorescence assay of intracellular lead ions. Chem Commun. 2017;53:7525–8.
Huang ZJ, Chen JM, Luo ZW, Wang XQ, Duan YX. Label-free and enzyme-free colorimetric detection of Pb2+ based on RNA cleavage and annealing-accelerated hybridization chain reaction. Anal Chem. 2019;91:4806–13.
Peng X, Liang WB, Wen ZB, **ong CY, Zheng YN, Chai YQ, et al. Ultrasensitive fluorescent assay based on a rolling-circle amplification-assisted multisite-strand-displacement-reaction signal-amplification strategy. Anal Chem. 2018;90:7474–9.
Tang SR, Tong P, Li H, Tang J, Zhang L. Ultrasensitive electrochemical detection of Pb2+ based on rolling circle amplification and quantum dots tagging. Biosens Bioelectron. 2013;42:608–11.
Li WY, Yang Y, Chen J, Zhang QF, Wang Y, Wang FY, et al. Detection of lead(II) ions with a DNAzyme and isothermal strand displacement signal amplification. Biosens Bioelectron. 2014;53:245–9.
Li Y, Qi XD, Ji XT, Guo YS. Simultaneous electrochemical determination of two analytes based on nuclease-assisted target recycling amplification. Anal Bioanal Chem. 2013;405:6845–51.
Wang WJ, Li JJ, Rui K, Gai PP, Zhang JR, Zhu JJ. Sensitive electrochemical detection of telomerase activity using spherical nucleic acids gold nanoparticles triggered mimic-hybridization chain reaction enzyme-free dual signal amplification. Anal Chem. 2015;87:3019–26.
Ge L, Wang WX, Hou T, Li F. A versatile immobilization-free photoelectrochemical biosensor for ultrasensitive detection of cancer biomarker based on enzyme-free cascaded quadratic amplification strategy. Biosens Bioelectron. 2016;77:220–6.
Wu RP, Zhu ZT, Xu XL, Yu CM, Li BL. An investigation of solid-state nanopores on label-free metal-ion signalling via the transition of RNA-cleavage DNAzyme and the hybridization chain reaction. Nanoscale. 2019;11:10339–47.
Chen JH, Zhou SG, Wen JL. Concatenated logic circuits based on a three-way DNA junction: a keypad-lock security system with visible readout and an automatic reset function. Angew Chem Int Ed. 2014;53:1–6.
Huang J, Wang H, Yang XH, Quan K, Yang YJ, Ying L, et al. Fluorescence resonance energy transfer-based hybridization chain reaction for in situ visualization of tumor-related mRNA. Chem Sci. 2016;7:3829–35.
Chen JH, Zhou SG. Label-free DNA Y junction for bisphenol A monitoring using exonuclease III-based signal protection strategy. Biosens Bioelectron. 2016;77:277–83.
Zhou DH, Wu W, Li Q, Pan JF, Chen JH. A label-free and enzyme-free aptasensor for visual Cd2+ detection based on split DNAzyme fragments. Anal Methods. 2019;11:3546–51.
Zhao JM, **g P, Xue SY, Xu WJ. Dendritic structure DNA for specific metal ion biosensor based on catalytic hairpin assembly and a sensitive synergistic amplification strategy. Biosens Bioelectron. 2017;87:157–63.
Song XL, Wang Y, Liu S, Zhang X, Wang JF, Wang HW, et al. A triply amplified electrochemical lead(II) sensor by using a DNAzyme and via formation of a DNA-gold nanoparticle network induced by a catalytic hairpin assembly. Microchim Acta. 2019;186:559.
Yun W, **ong W, Wu H, Fu M, Huang Y, Liu XY, et al. Graphene oxide-based fluorescent “turn-on” strategy for Hg2+ detection by using catalytic hairpin assembly for amplification. Sensors Actuators B. 2017;249:493–8.
Ge L, Wang WX, Sun XM, Hou T, Li F. Affinity-mediated homogeneous electrochemical aptasensor on graphene platform for ultrasensitive biomolecule detection via exonuclease-assisted target-analog recycling amplification. Anal Chem. 2016;88(4):2212–9.
Wang WX, Ge L, Sun XM, Hou T, Li F. Graphene-assisted label-free homogeneous electrochemical biosensing strategy based on aptamer-switched bidirectional DNA polymerization. ACS Appl Mater Interfaces. 2015;7:28566–75.
Lu C, Huang PJ, Liu BW, Ying YB, Liu JW. Comparison of graphene oxide and reduced graphene oxide for DNA adsorption and sensing. Langmuir. 2016;32:10776–83.
He SJ, Song B, Li D, Zhu CF, Qi WP, Wen YQ, et al. A graphene nanoprobe for rapid, sensitive, and multicolor fluorescent DNA analysis. Adv Funct Mater. 2010;20:453–9.
Wang YH, Deng HH, Liu YH, Shi XQ, Liu AL, Peng HP, et al. Partially reduced graphene oxide as highly efficient DNA nanoprobe. Biosens Bioelectron. 2016;80:140–5.
Noordeen AK, Sambasivam S, Chinnasamy S, Ramasamy J, Subramani T. Hierarchical flower structured Bi2S3/reduced graphene oxide nanocomposite for high electrochemical performance. J Inorg Organomet Polym. 2018;28:73–83.
Li X, **e JQ, Jiang BY, Yuan R, **ang Y. Metallo-toehold-activated catalytic hairpin assembly formation of three-way DNAzyme junctions for amplified fluorescent detection of Hg2+. ACS Appl Mater Interfaces. 2017;9:5733–8.
Long GL, Winefordner JD. Limit of detection. A closer look at the IUPAC definition. Anal. Chem. 1983;55:712A–24A.
Jiang MH, Lu P, Lei YM, Chai YQ, Yuan R, Zhuo Y. Self-accelerated electrochemiluminescence emitters of Ag@SnO2 nanoflowers for sensitive detection of cardiac troponin T. Electrochim Acta. 2018;271(1):464–71.
Park M, Ha HD, Kim YT, Jung JH, Kim SH, Kim DH, et al. Combination of a sample pretreatment microfluidic device with a photoluminescent graphene oxide quantum dot sensor for trace lead detection. Anal Chem. 2015;87:10969–75.
Zhou WJ, Liang WB, Li DX, Yuan R, **ang Y. Dual-color encoded DNAzyme nanostructures for multiplexed detection of intracellular metal ions in living cells. Biosens Bioelectron. 2016;85:573–9.
Liu SY, Na WD, Pang S, Su XG. Fluorescence detection of Pb2+ based on the DNA sequence functionalized CdS quantum dots. Biosens Bioelectron. 2014;58:17–21.
Li X, Yun W, Guo WL, Zhang WL, Yang LZ. Simple, one step and sensitive fluorescent nanoprobe for simultaneous detection of Pb2+ and Cu2+ based on pincer enzyme strand and signal amplification strategy. Sensors Actuators B Chem. 2019;301:127170.
Lu W, Lin CQ, Yang J, Wang XQ, Yao B, Wang M. A DNAzyme assay coupled with effective magnetic separation and rolling circle amplification for detection of lead cations with a smartphone camera. Anal Bioanal Chem. 2019;411:5383–91.
Wu JK, Lu YF, Ren NN, Jia M, Wang RN, Zhang JL. DNAzyme-functionalized R-phycoerythrin as a cost-effective and environment-friendly fluorescent biosensor for aqueous Pb2+ detection. Sensors. 2019;19:2732.
Funding
This work was supported by the National Natural Science Foundation of China (21775122, 21775124); the Natural Science Foundation of Chongqing City (cstc2018icyjAX0693), China; the Natural Science Foundation of Jiangsu Province of China (BK20170378); and the Natural Science Research Foundation of Jiangsu Higher Education Institutions (17KJB150036).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 976 kb)
Rights and permissions
About this article
Cite this article
Wang, J., Chen, S., Yuan, R. et al. DNA branched junctions induced the enhanced fluorescence recovery of FAM-labeled probes on rGO for detecting Pb2+. Anal Bioanal Chem 412, 2455–2463 (2020). https://doi.org/10.1007/s00216-020-02458-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-020-02458-2