Abstract
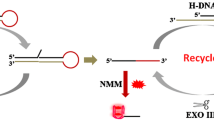
A novel enzyme-linked aptamer assay (ELAA) with the aid of Exonuclease I (Exo I) for colorimetric detection of small molecules was developed. The fluorescein isothiocyanate (FITC)-labeled aptamer was integrated into a double-stranded DNA (dsDNA). In the presence of target, the binding of aptamer with target protected the aptamer from Exo I degradation, which resulted in the FITC tag remaining on the aptamer. Then, the anti-FITC-HRP conjugate was used to produce an optically observable signal. By monitoring the color change, we were able to detect two model molecules, ATP and L-argininamide, with high selectivity and high sensitivity even in the serum matrix. It is expected to be a simple and general ELAA method with wide applicability.

Sensing strategy for exonuclease I-aided enzyme-linked aptamer assay






Similar content being viewed by others
References
Tortorano AM, Esposto MC, Prigitano A, Grancini A, Ossi C, Cavanna C, Cascio GL (2012) Cross-reactivity of Fusarium spp. in the Aspergillus galactomannan enzyme-linked immunosorbent assay. J Clin Microbiol 50:1051–1053
Liao ND, Lee WY (2012) Detection of carbonic anhydrase IX protein in the diagnosis of malignant pleural effusion by enzyme-linked immunosorbent assay and immunocytochemistry. Cancer Cytopathol 120(4):269–275
Sapir A, Shalev AH, Skalka N, Bronshtein A, Altstein M (2013) Development of an enzyme-linked immunosorbent assay and a beta-I adrenergic receptor-based assay for monitoring the drug atenolol. Environ Toxicol Chem 32(3):585–593
Irenge LM, Gala JL (2012) Rapid detection methods for Bacillus anthracis in environmental samples: a review. Appl Microbiol Biotechnol 93:1411–1422
Fang X, Tan W (2010) Aptamers generated from cell-SELEX for molecular medicine: a chemical biology approach. Acc Chem Res 43(1):48–57
Yang CJ, Jockusch S, Vicens M, Turroand NJ, Tan W (2005) Light-switching excimer probes for rapid protein monitoring in complex biological fluids. Proc Natl Acad Sci U S A 102(48):17278–17283
Tan X, Chen T, **ong X, Mao Y, Zhu G, Yasun E, Li C, Zhu Z, Tan W (2012) Semiquantification of ATP in Live cells using nonspecific desorption of DNA from graphene oxide as the internal reference. Anal Chem 84(20):8622–8627
Wang Y, Bao L, Liu Z, Pang D (2011) Aptamer biosensor based on fluorescence resonance energy transfer from upconverting phosphors to carbon nanoparticles for thrombin detection in human plasma. Anal Chem 83(21):8130–8137
Luo F, Zheng L, Chen S, Cai Q, Lin Z, Qiu B, Chen G (2012) An aptamer-based fluorescence biosensor for multiplex detection using unmodified gold nanoparticles. Chem Commun 48:6387–6389
Zhu Z, Wu C, Liu H, Zou Y, Zhang X, Kang H, Yang CJ, Tan W (2010) An aptamer cross-linked hydrogel as a colorimetric platform for visual detection. Angew Chem 122(6):1070–1074
Li J, Fu HE, Wu LJ, Zheng AX, Chen GN, Yang HH (2012) General colorimetric detection of proteins and small molecules based on cyclic enzymatic signal amplification and hairpin aptamer probe. Anal Chem 84(12):5309–5315
Wei H, Li B, Li J, Wang E, Dong S (2007) Simple and sensitive aptamer-based colorimetric sensing of protein using unmodified gold nanoparticle probes. Chem Commun : 3735–3737
**a F, Zuo X, Yang R, **ao Y, Kang D, Vallee-Belisle A, Gong X, Yuen JD, Hsu BBY, Heeger AJ, Plaxco KW (2010) Colorimetric detection of DNA, small molecules, proteins, and ions using unmodified gold nanoparticles and conjugated polyelectrolytes. Proc Natl Acad Sci U S A 107(24):10837–10841
**ao Y, Piorek BD, Plaxco KW, Heeger AJ (2005) A reagentless signal-on architecture for electronic, aptamer-based sensors via target-induced strand displacement. J Am Chem Soc 127(51):17990–17991
Zuo X, Song S, Zhang J, Pan D, Wang L, Fan C (2007) A target-responsive electrochemical aptamer switch (TREAS) for reagentless detection of nanomolar ATP. J Am Chem Soc 129(5):1042–1043
Li B, Du Y, Wei H, Dong S (2007) Reusable, label-free electrochemical aptasensor for sensitive detection of small molecules. Chem Commun: 3780–3782
Yin BC, Guan YM, Ye BC (2012) An ultrasensitive electrochemical DNA sensor based on the ssDNA-assisted cascade of hybridization reaction. Chem Commun 48:4208–4210
Huang H, Tan Y, Shi J, Liang G, Zhu JJ (2010) DNA aptasensor for the detection of ATP based on quantum dots electrochemiluminescence. Nanoscale 2:606–612
Lin Z, Luo F, Liu Q, Chen L, Qiu B, Cai Z, Chen G (2011) Signal-on electrochemiluminescent biosensor for ATP based on the recombination of aptamer chip. Chem Commun 47:8064–8066
Baldrich E, Acero JL, Reekmans G, Laureyn W, O’ Sullivan CK (2005) Displacement enzyme linked aptamer assay. Anal Chem 77(15):4774–4784
Higuchi A, Siao YD, Yang ST, Hsieh PV, Fukushima H, Chang Y, Ruaan RC, Chen WY (2008) Preparation of a DNA aptamer—Pt complex and its use in the colorimetric sensing of thrombin and anti-thrombin antibodies. Anal Chem 80(17):6580–6586
Sharma AK, Kent AD, Heemstra JM (2012) Enzyme-linked small-molecule detection using split aptamer ligation. Anal Chem 84(14):6104–6109
Nie J, Deng Y, Deng QP, Zhang DW, Zhang XX, Zhou YL (2013) A self-assemble aptamer fragment/target complex based high-throughput colorimetric aptasensor using enzyme linked aptamer assay. Talanta 106:309–314
Park H, Paeng IR (2011) Development of direct competitive enzyme-linked aptamer assay for determination of dopamine in serum. Anal Chim Acta 685(1):65–73
Barthelmebs L, Jonca J, Prieto-Simon B, Marty JL (2011) Enzyme-linked aptamer assays (ELAAs), based on a competition format for a rapid and sensitive detection of ochratoxin A in wine. Food Control 22(5):737–743
Yuan M, Zhu Y, Lou X, Chen C, Wei G, Lan M, Zhao J (2012) Sensitive label-free oligonucleotide-based microfluidic detection of mercury (II) ion by using exonuclease I. Biosens Bioelectron 31(1):330–336
Zheng D, Zou R, Lou X (2012) Label-free fluorescent detection of ions, proteins, and small molecules using structure-switching aptamers, SYBR gold, and exonuclease I. Anal Chem 84(8):3554–3560
Jiang B, Wang M, Li C, **e J (2013) Label-free and amplified aptasensor for thrombin detection based on background reduction and direct electron transfer of hemin. Biosens Bioelectron 43:289–392
Wang XL, Li F, Su YH, Sun X, Li XB, Schluesener HJ, Tang F, Xu SQ (2004) Ultrasensitive detection of protein using an aptamer-based exonuclease protection assay. Anal Chem 76(19):5605–5610
Huizenga DE, Szostak JW (1995) A DNA aptamer that binds adenosine and ATP. Biochemistry 34(2):656–665
Kong L, Xu J, Xu Y, **ang Y, Yuan R, Chai Y (2013) A universal and label-free aptasensor for fluorescent detection of ATP and thrombin based on SYBR green I dye. Biosens Bioelectron 42:193–197
He X, Li Z, Jia X, Wang K, Yin J (2013) A highly selective sandwich-type FRET assay for ATP detection based on silica coated photon upconverting nanoparticles and split aptamer. Talanta 111:105–110
Zhou Z, Du Y, Dong S (2011) Double-strand DNA-templated formation of copper nanoparticles as fluorescent probe for label-free aptamer sensor. Anal Chem 83(13):5122–5127
Wang J, Wang L, Liu X, Liang Z, Song S, Li W, Li G, Fan C (2007) A gold nanoparticle-based aptamer target binding readout for ATP assay. Adv Mater 19(22):3943–3946
Liu X, Freeman R, Golub E, Willner I (2011) Chemiluminescence and chemiluminescence resonance energy transfer (CRET) aptamer sensors using catalytic hemin/G-quadruplexes. ACS Nano 5(9):7648–7655
Cruz-Aguado JA, Chen Y, Zhang Z, Elowe NH, Brook MA, Brennan JD (2004) Ultrasensitive ATP detection using firefly luciferase entrapped in sugar-modified sol–gel-derived silica. JAM CHEM SOC 2004(126):6878–6879
Ozaki H, Nishihira A, Wakabayashi M, Kuwahara M, Sawai H (2006) Biomolecular sensor based on fluorescence-labeled aptamer. Bioorg Med Chem Lett 16(16):4381–4384
Zhu Z, Yang C, Zhou X, Qin J (2011) Label-free aptamer-based sensors for L-argininamide by using nucleic acid minor groove binding dyes. Chem Commun 47:3192–3194
Song KM, Cho M, Jo H, Min K, Jeon SH, Kim T, Han MS, Ku JK, Ban C (2011) Gold nanoparticle-based colorimetric detection of kanamycin using a DNA aptamer. Anal Biochem 415(2):175–181
Mei H, Bing T, Yang X, Qi C, Chang T, Liu X, Cao Z, Shangguan D (2012) Functional-group specific aptamers indirectly recognizing compounds with alkyl amino group. Anal Chem 84(17):7323–7329
Stojanovic MN, de Prada P, Landry DW (2000) Fluorescent sensors based on aptamer self-assembly. J Am Chem Soc 122:11547–11548
Kato T, Takemura T, Yano K, Ikebukuro K, Karube I (2000) In vitro selection of DNA aptamers which bind to cholic acid. Biochim Biophys Acta 1493(1–2):12–18
Qi C, Bing T, Mei H, Yang X, Liu X, Shangguan D (2013) G-quadruplex DNA aptamers for zeatin recognizing. Biosens Bioelectron 41:157–162
Acknowledgments
This work was supported by National Basic Research Program of China (2011C91101, 2013CB933701), NSFC (no. 21127901, 21121063), and Chinese Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, Q., Zhang, Z., Xu, L. et al. Exonuclease I aided enzyme-linked aptamer assay for small-molecule detection. Anal Bioanal Chem 406, 2949–2955 (2014). https://doi.org/10.1007/s00216-014-7705-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-014-7705-z