Abstract
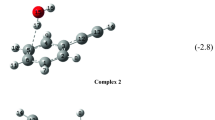
An interaction potential previously developed for the acetylene–polyyne dimer was used to explore the interaction potential surfaces for clusters containing a diacetylene molecule and two or more acetylene molecules. Ab initio calculations were performed on the smallest clusters in order to assess the energetic and structural features predicted by the model potential. The preferred arrangements of the monomers in the clusters maximize the favorable quadrupole–quadrupole interactions between the monomers.
Similar content being viewed by others
Acknowledgments.
This work was supported, in part, by a grant from the Physical Chemistry Program of the National Science Foundation (CHE-0131932).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chenoweth, K., Dykstra, C. Diacetylene's weak bonding to acetylene clusters. Theor Chem Acc 110, 100–104 (2003). https://doi.org/10.1007/s00214-003-0458-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00214-003-0458-y