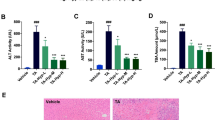
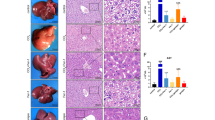
Abstract
Pyrrolizidine alkaloids (PAs) are one of the most significant groups of hepatotoxic phytotoxins. It is well-studied that metabolic activation of PAs generates reactive pyrrolic metabolites that rapidly bind to cellular proteins to form pyrrole–protein adducts leading to hepatotoxicity. Pyrrole–protein adducts all contain an identical core pyrrole moiety regardless of structures of the different PAs; however, the proteins forming pyrrole–protein adducts are largely unknown. The present study revealed that ATP synthase subunit beta (ATP5B), a critical subunit of mitochondrial ATP synthase, was a protein bound to the reactive pyrrolic metabolites forming pyrrole–ATP5B adduct. Using both anti-ATP5B antibody and our prepared anti-pyrrole–protein antibody, pyrrole–ATP5B adduct was identified in the liver of rats, hepatic sinusoidal endothelial cells, and HepaRG hepatocytes treated with retrorsine, a well-studied representative hepatotoxic PA. HepaRG cells were then used to further explore the consequence of pyrrole–ATP5B adduct formation. After treatment with retrorsine, significant amounts of pyrrole–ATP5B adduct were formed in HepaRG cells, resulting in remarkably reduced ATP synthase activity and intracellular ATP level. Subsequently, mitochondrial membrane potential and respiration were reduced, leading to mitochondria-mediated apoptotic cell death. Moreover, pre-treatment of HepaRG cells with a mitochondrial membrane permeability transition pore inhibitor significantly reduced retrorsine-induced toxicity, further revealing that mitochondrial dysfunction caused by pyrrole–ATP5B adduct formation significantly contributed to PA intoxication. Our findings for the first time identified ATP5B as a protein covalently bound to the reactive pyrrolic metabolites of PAs to form pyrrole–ATP5B adduct, which impairs mitochondrial function and significantly contributes to PA-induced hepatotoxicity.







Similar content being viewed by others

References
Antoniel M, Giorgio V, Fogolari F, Glick GD, Bernardi P, Lippe G (2014) The oligomycin-sensitivity conferring protein of mitochondrial ATP synthase: emerging new roles in mitochondrial pathophysiology. Int J Mol Sci 15(5):7513–7536. https://doi.org/10.3390/ijms15057513
Avril A, Pichard V, Bralet MP, Ferry N (2004) Mature hepatocytes are the source of small hepatocyte-like progenitor cells in the retrorsine model of liver injury. J Hepatol 41(5):737–743. https://doi.org/10.1016/j.jhep.2004.07.029
Begriche K, Massart J, Robin MA, Borgne-Sanchez A, Fromenty B (2011) Drug-induced toxicity on mitochondria and lipid metabolism: mechanistic diversity and deleterious consequences for the liver. J Hepatol 54(4):773–794. https://doi.org/10.1016/j.jhep.2010.11.006
Bonora M, Wieckowsk MR, Chinopoulos C et al (2015) Molecular mechanisms of cell death: central implication of ATP synthase in mitochondrial permeability transition (vol 34, pg 1475, 2015). Oncogene 34(12):1608–1608. https://doi.org/10.1038/onc.2014.462
Bordet T, Berna P, Abitbol J-L, Pruss RM (2010) Olesoxime (TRO19622): A novel mitochondrial-targeted neuroprotective compound. Pharmaceuticals 3(2):345–368. https://doi.org/10.3390/ph3020345
Brand Martin D, Nicholls David G (2011) Assessing mitochondrial dysfunction in cells. Biochem J 435(Pt 2):297–312. https://doi.org/10.1042/BJ20110162
Chojkier M (2003) Hepatic sinusoidal-obstruction syndrome: toxicity of pyrrolizidine alkaloids. J Hepatol 39(3):437–446. https://doi.org/10.1016/S0168-8278(03)00231-9
Danial NN, Korsmeyer SJ (2004) Cell death: critical control points. Cell 116(2):205–219. https://doi.org/10.1016/S0092-8674(04)00046-7
DeLeve LD, Wang X, Kuhlenkamp JF, Kaplowitz N (1996) Toxicity of azathioprine and monocrotaline in murine sinusoidal endothelial cells and hepatocytes: the role of glutathione and relevance to hepatic venoocclusive disease. Hepatology 23(3):589–599. https://doi.org/10.1002/hep.510230326
DeLeve LD, Wang XD, Tsai J, Kanel G, Strasberg S, Tokes ZA (2003) Sinusoidal obstruction syndrome (veno-occlusive disease) in the rat is prevented by matrix metalloproteinase inhibition. Gastroenterology 125(3):882–890. https://doi.org/10.1016/S0016-5085(03)01056-4
Divakaruni AS, Paradyse A, Ferrick DA, Murphy AN, Jastroch M (2014) Analysis and interpretation of microplate-based oxygen consumption and pH data. Methods Enzymol 547:309–354.https://doi.org/10.1016/B978-0-12-801415-8.00016-3
Edgar JA, Colegate SM, Boppre M, Molyneux RJ (2011) Pyrrolizidine alkaloids in food: a spectrum of potential health consequences. Food Addit Contam A 28(3):308–324. https://doi.org/10.1080/19440049.2010.547520
Edgar JA, Molyneux RJ, Colegate SM (2015) Pyrrolizidine alkaloids: potential role in the etiology of cancers, pulmonary hypertension, congenital anomalies, and liver Disease. Chem Res Toxicol 28(1):4–20. https://doi.org/10.1021/tx500403t
Fu PP (2017) Pyrrolizidine alkaloids: metabolic activation pathways leading to liver tumor initiation. Chem Res Toxicol 30(1):81–93. https://doi.org/10.1021/acs.chemrestox.6b00297
Fu PP, **a Q, Lin G, Chou MW (2004) Pyrrolizidine alkaloids–genotoxicity, metabolism enzymes, metabolic activation, and mechanisms. Drug Metab Rev 36(1):1–55. https://doi.org/10.1081/DMR-120028426
Gouarne C, Giraudon-Paoli M, Seimandi M et al (2013) Olesoxime protects embryonic cortical neurons from camptothecin intoxication by a mechanism distinct from BDNF. Br J Pharmacol 168(8):1975–1988. https://doi.org/10.1111/bph.12094
Hart SN, Li Y, Nakamoto K, Subileau EA, Steen D, Zhong XB (2010) A comparison of whole genome gene expression profiles of HepaRG Cells and HepG2 cells to primary human hepatocytes and human liver tissues. Drug Metab Dispos 38(6):988–994. https://doi.org/10.1124/dmd.109.031831
Hüttemann M, Lee I, Pecinova A, Pecina P, Przyklenk K, Doan JW (2008) Regulation of oxidative phosphorylation, the mitochondrial membrane potential, and their role in human disease. J Bioenerg Biomembr 40(5):445. https://doi.org/10.1007/s10863-008-9169-3
Ji LL, Liu TY, Chen Y, Wang ZT (2009) Protective mechanisms of N-acetyl-cysteine against pyrrolizidine alkaloid clivorine-induced hepatotoxicity. J Cell Biochem 108(2):424–432. https://doi.org/10.1002/jcb.22269
Jonckheere AI, Smeitink JAM, Rodenburg RJT (2012) Mitochondrial ATP synthase: architecture, function and pathology. J Inherit Metab Dis 35(2):211–225. https://doi.org/10.1007/s10545-011-9382-9
Kantari C, Walczak H (2011) Caspase-8 and Bid: caught in the act between death receptors and mitochondria. Bba-Mol Cell Res 1813(4):558–563. https://doi.org/10.1016/j.bbamcr.2011.01.026
Kolrep F, Numata J, Kneuer C et al (2017) In vitro biotransformation of pyrrolizidine alkaloids in different species. Part I: Microsomal degradation. Arch Toxicol. https://doi.org/10.1007/s00204-017-2114-7
Li YH, Tai WC, Xue JY et al (2015) Proteomic study of pyrrolizidine alkaloid-induced hepatic sinusoidal obstruction syndrome in rats. Chem Res Toxicol 28(9):1715–1727. https://doi.org/10.1021/acs.chemrestox.5b00113
Li N, Zhang F, Lian W, Wang H, Zheng J, Lin G (2017) Immunoassay approach for diagnosis of exposure to pyrrolizidine alkaloids. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. https://doi.org/10.1080/10590501.2017.1328828
Lin G, Cui YY, Hawes EM (2000) Characterization of rat liver microsomal metabolites of clivorine, an hepatotoxic otonecine-type pyrrolizidine alkaloid. Drug Metab Dispos 28(12):1475–1483
Lin G, Wang JY, Li N et al (2011) Hepatic sinusoidal obstruction syndrome associated with consumption of Gynura segetum. J Hepatol 54(4):666–673. https://doi.org/10.1016/j.jhep.2010.07.031
Lübberstedt M, Müller-Vieira U, Mayer M et al (2011) HepaRG human hepatic cell line utility as a surrogate for primary human hepatocytes in drug metabolism assessment in vitro. J Pharmacol Toxicol Methods 63(1):59–68. https://doi.org/10.1016/j.vascn.2010.04.013
Marroquin LD, Hynes J, Dykens JA, Jamieson JD, Will Y (2007) Circumventing the Crabtree effect: replacing media glucose with galactose increases susceptibility of HepG2 cells to mitochondrial toxicants. Toxicol Sci 97(2):539–547. https://doi.org/10.1093/toxsci/kfm052
Mattocks AR (1968) Toxicity of pyrrolizidine alkaloids. Nature 217(5130):723–728. https://doi.org/10.1038/217723a0
Mattocks AR (1986) Chemistry and toxicology of pyrrolizidine alkaloids. Academic Press, London
Mayer F, Luthy J (1993) Heliotrope poisoning in Tajikistan. Lancet 342(8865):246–247. https://doi.org/10.1016/0140-6736(93)92341-P
McGill MR, Yan HM, Ramachandran A, Murray GJ, Rollins DE, Jaeschke H (2011) HepaRG cells: a human model to study mechanisms of acetaminophen hepatotoxicity. Hepatology 53(3):974–982. https://doi.org/10.1002/hep.24132
McLelland GL, Soubannier V, Chen CX, McBride HM, Fon EA (2014) Parkin and PINK1 function in a vesicular trafficking pathway regulating mitochondrial quality control. EMBO J 33(4):282–295. https://doi.org/10.1002/embj.201385902
Mingatto FE, Dorta DJ, dos Santos AB et al (2007) Dehydromonocrotaline inhibits mitochondrial complex I. A potential mechanism accounting for hepatotoxicity of monocrotaline. Toxicon 50(5):724–730. https://doi.org/10.1016/j.toxicon.2007.06.006
Mohabbat O, Srivastava RN, Younos MS, Merzad AA, Sediq GG, Aram GN (1976) Outbreak of hepatic veno-occlusive disease. Northwestern Afghanistan Lancet 2(7980):269–271. https://doi.org/10.1016/S0140-6736(76)90726-1
Prakash AS, Pereira TN, Reilly PEB, Seawright AA (1999) Pyrrolizidine alkaloids in human diet. Mutat Res-Gen Tox En 443(1–2):53–67. https://doi.org/10.1016/S1383-5742(99)00010-1
Robinson O, Want E, Coen M et al (2014) Hirmi Valley liver disease: a disease associated with exposure to pyrrolizidine alkaloids and DDT. J Hepatol 60(1):96–102. https://doi.org/10.1016/j.jhep.2013.07.039
Ruan J, Yang M, Fu P, Ye Y, Lin G (2014) Metabolic activation of pyrrolizidine alkaloids: insights into the structural and enzymatic basis. Chem Res Toxicol 27(6):1030–1039. https://doi.org/10.1021/tx500071q
Ruan JQ, Gao H, Li N et al (2015) Blood pyrrole–protein adducts—a biomarker of pyrrolizidine alkaloid-induced liver injury in humans. J Environ Sci Heal C 33(4):404–421. https://doi.org/10.1080/10590501.2015.1096882
Scott ID, Nicholls DG (1980) Energy transduction in intact synaptosomes. Influence of plasma-membrane depolarization on the respiration and membrane potential of internal mitochondria determined in situ. Biochem J 186(1):21–33
Swiss R, Will Y (2011) Assessment of mitochondrial toxicity in HepG2 cells cultured in high-glucose- or galactose-containing media. Curr Protoc Toxicol Chap. 2(Unit 2):20. https://doi.org/10.1002/0471140856.tx0220s49
Tait SW, Green DR (2013) Mitochondrial regulation of cell death. Cold Spring Harbor Perspect Biol 5:a008706–a008706. https://doi.org/10.1101/cshperspect.a008706
Tandon BN, Tandon RK, Tandon HD, Narndranathan M, Joshi YK (1976) Epidemic of Veno-occlusive disease of liver in central India. Lancet 2(7980):271–272. https://doi.org/10.1016/S0140-6736(76)90727-3
Willmot FC, Robertson GW (1920) Senecio disease or cirrhosis of the liver, due to senecio poisoning. Lancet 196(5069):848–849. https://doi.org/10.1016/S0140-6736(01)00020-4
**a QS, Zhao YW, Lin G, Beland FA, Cai LN, Fu PP (2016) Pyrrolizidine alkaloid-protein adducts: potential non-invasive biomarkers of pyrrolizidine alkaloid-induced liver toxicity and exposure. Chem Res Toxicol 29(8):1282–1292. https://doi.org/10.1021/acs.chemrestox.6b00120
Yang M, Ruan J, Fu PP, Lin G (2016) Cytotoxicity of pyrrolizidine alkaloid in human hepatic parenchymal and sinusoidal endothelial cells: firm evidence for the reactive metabolites mediated pyrrolizidine alkaloid-induced hepatotoxicity. Chem Biol Interact 243:119–126. https://doi.org/10.1016/j.cbi.2015.09.011
Yang M, Ruan J, Gao H et al (2017) First evidence of pyrrolizidine alkaloid N-oxide-induced hepatic sinusoidal obstruction syndrome in humans. Arch Toxicol 91(12):3913–3925. https://doi.org/10.1007/s00204-017-2013-y
Yeong ML, Wakefield SJ, Ford HC (1993) Hepatocyte membrane injury and bleb formation following low-dose comfrey toxicity in rats. Int J Exp Pathol 74(2):211–217
Zheng J, Ramirez VD (2000) Inhibition of mitochondrial proton F0F1-ATPase/ATP synthase by polyphenolic phytochemicals. Br J Pharmacol 130(5):1115–1123. https://doi.org/10.1038/sj.bjp.0703397
Zhuge Y, Wang Y, Zhang F et al (2018) Clinical characteristics and treatment of Pyrrolizidine alkaloid-related hepatic vein occlusive disease. Liver Int. https://doi.org/10.1111/liv.13684
Acknowledgements
We sincerely thank Drs. Christiane Guguen-Guillouzo and Philippe Gripon from U522 INSERM and Dr. Christian Trepo from U271 INSERM for providing HepaRG cells.
Funding
The present study was supported by Research Grant Council of Hong Kong (GRF Grants nos. 14111816 and 14110714) and CUHK Direct Grant (4054302).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they do not have anything to disclose regarding funding or conflict of interest with respect to this manuscript. This article is not an official U.S. Food and Drug Administration guidance or policy statement. No official support or endorsement by U.S Food and Drug Administration is intended or should be inferred.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lu, Y., Ma, J., Song, Z. et al. The role of formation of pyrrole–ATP synthase subunit beta adduct in pyrrolizidine alkaloid-induced hepatotoxicity. Arch Toxicol 92, 3403–3414 (2018). https://doi.org/10.1007/s00204-018-2309-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-018-2309-6