Abstract.
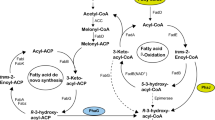
In order to investigate the role of the putative epimerase function of the β-oxidation multienzyme complex (FadBA) in the provision of (R)-3-hydroxyacyl-CoA thioesters for medium-chain-length polyhydroxyalkanoate (PHAMCL) biosynthesis, the fadBA Po operon of Pseudomonas oleovorans was cloned and characterized. The fadBA Po operon and a class-II PHA synthase gene of Pseudomonas aeruginosa were heterologously co-expressed in Escherichia coli to determine whether the putative epimerase function of FadBAPo has the ability to provide precursors for PHA accumulation in a non-PHA-accumulating bacterium. Cultivation studies with fatty acids as carbon source revealed that FadBAPo did not mediate PHAMCL biosynthesis in the E. coli wild-type strain harboring a PHA synthase gene. However, PHA accumulation was strongly impaired in a recombinant E. coli fadB mutant, which harbored a PHA synthase gene. These data indicate that in pseudomonads FadBA does not possess the inherent property, based on a putative epimerase function, to provide the (R)-enantiomer of 3-hydroxyacyl-CoA efficiently and that other linking enzymes are required to efficiently channel intermediates of β-oxidation towards PHAMCL biosynthesis. However, the phaJ gene from P. oleovorans and from Pseudomonas putida, both of which encoded a 3-Re enoyl-CoA hydratase, was identified. The co-expression of phaJ Po/Pp with either a class-II PHA synthase gene or the PHA synthase gene from Aeromonas punctata in E. coli revealed that PhaJPo/Pp mediated biosynthesis of either PHAMCL, contributing to about 1% of cellular dry mass, or of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate), contributing to 3.6% of cellular dry mass, when grown on decanoate. These data indicate that FadBAPo does not mediate the provision of (R)-3-hydroxyacyl-CoA, which resembles FadBA of non-PHA-accumulating bacteria, and that 3-Re enoyl-CoA hydratases are required to divert intermediates of fatty acid β-oxidation towards PHA biosynthesis in P. oleovorans.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Electronic Publication
Rights and permissions
About this article
Cite this article
Fiedler, S., Steinbüchel, A. & Rehm, B.H. The role of the fatty acid β-oxidation multienzyme complex from Pseudomonas oleovorans in polyhydroxyalkanoate biosynthesis: molecular characterization of the fadBA operon from P. oleovorans and of the enoyl-CoA hydratase genes phaJ from P. oleovorans and Pseudomonas putida. Arch Microbiol 178, 149–160 (2002). https://doi.org/10.1007/s00203-002-0444-0
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s00203-002-0444-0