Abstract
Summary
Spinal cord injury (SCI) results in impaired fracture healing in mice while leading to significant bone loss. Poor fracture healing following SCI is consistent with significant bone loss.
Introduction
SCI leads to significant bone loss in sublesional limbs, but there is few data concerning the relationship between fracture healing and bone loss following SCI. This study was undertaken to investigate the effect of SCI on fracture healing using a mouse femur fracture model.
Methods
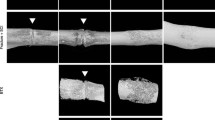
One hundred twenty male C57BL/6J mice were randomly divided into SCI and control groups (n = 60, respectively). A femoral shaft fracture was generated and fixed with intramedullary pins 3 weeks after SCI. Fracture healing was evaluated by micro-computed tomography (micro-CT) for callus formation and mineralization and neovascularization, and bone mineral density (BMD) was measured by DXA at 1, 2, and 4 weeks after fracture. Serum vascular endothelial growth factor (VEGF), osteocalcin, and alkaline phosphatase (ALP) were assessed using ELISA at each time point. Biomechanical testing was performed at 2 and 4 weeks.
Results
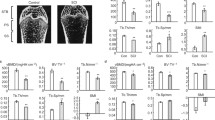
BMD in SCI mice was significantly lower compared to control mice at each time point, with callus volume and all vessel parameters reduced as measured by micro-CT. Ultimate stress of the femora was significantly lower in SCI mice than in control mice at 2 and 4 weeks after fracture, whereas Young's modulus between the SCI and control mice turned to be significantly different at 4 weeks. Serum VEGF was lower in SCI mice than in the control group at 2 and 4 weeks, whereas serum osteocalcin and ALP were lower in SCI mice than in control ones at each time point.
Conclusion
Significant bone loss and fracture healing impairment was noted in SCI mice. Decreased angiogenesis is consistent with the changes of microarchitecture and biomechanical properties during fracture healing.





Similar content being viewed by others
References
Biering-Sorensen F, Bohr H, Schaadt O (1988) Bone mineral content of the lumbar spine and lower extremities years after lesion. Paraplegia 26:293–301
Garland DE, Adkins RH, Stewart CA, Ashford R, Vigil D (2001) Regional osteoporosis in women who have a complete spinal cord injury who have a complete spinal cord injury. J Bone Joint Surg Am 83:1195–1200
Leblanc AD, Schneider VS, Evans HJ, Engelbretson DA, Krebs JM (1990) Bone mineral loss and recovery after 17 weeks of bed rest. J Bone Miner Res 5:843–850
Jiang SD, Jiang LS, Dai LY (2006) Spinal cord injury causes more damage to bone mass, bone structure, biomechanical properties and bone metabolism than sciatic neurectomy in young rats. Osteoporos Int 17:1552–1561
Jiang SD, Shen C, Jiang LS, Dai LY (2007) Differences of bone mass and bone structure in osteopenic rat models caused by spinal cord injury and ovariectomy. Osteoporos Int 18:743–750
Liu D, Zhao CQ, Li H, Jiang SD, Jiang LS, Dai LY (2008) Effects of spinal cord injury and hindlimb immobilization on sublesional and supralesional bones in young growing rats. Bone 43:119–125
Jones LM, Legge M, Goulding A (2002) Intensive exercise may preserve bone mass of the upper limbs in spinal cord injured males but does not retard demineralisation of the lower body. Spinal Cord 40:230–235
Bloomfield SA, Mysiw WJ, Jackson RD (1996) Bone mass and endocrine adaptations to training in spinal cord injured individuals. Bone 19:61–68
Gilchrist NL, Frampton CM, Acland RH, Nicholls MG, March RL, Maguire P, Heard A, Reilly P, Marshall K (2007) Alendronate prevents bone loss in patients with acute spinal cord injury: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab 92:1385–1390
Jiang SD, Jiang LS, Dai LY (2007) Effects of spinal cord injury on osteoblastogenesis, osteoclastogenesis and gene expression profiling in osteoblasts in young rats. Osteoporos Int 18:339–349
Comarr AE, Hutchinson RH, Bors E (1962) Extremity fractures of patients with spinal cord injuries. Am J Surg 103:32–739
Ragnarsson KT, Sell GH (1981) Lower extremity fractures after spinal cord injury: a retrospective study. Arch Phys Med Rehabil 62:418–423
Ingram RR, Suman RK, Freeman PA (1989) Lower limb fractures in the chronic spinal cord injured patient. Paraplegia 27:133–139
Vestergaard P, Krogh K, Rejnmark L, Mosekilde L (1998) Fracture rates and risk factors for fractures in patients with spinal cord injury. Spinal Cord 36:790–796
Lazo MG, Shirazi P, Sam M, Giobbie-Hurder A, Blacconiere MJ, Muppidi M (2001) Osteoporosis and risk of fracture in men with spinal cord injury. Spinal Cord 39:208–214
Miyamoto T (1987) An experimental study on fracture healing in paraplegic rats. Nippon Seikeigeka Gakkai Zasshi 61:1135–1145, in Japanese
Aro H, Eerola E, Aho AJ, Penttinen R (1981) Healing of experimental fractures in the denervated limbs of the rat. Clin Orthop 155:211–217
Aro H, Eerola E, Aho AJ (1985) Fracture healing in paraplegic rats. Acta Orthop Scand 56:228–232
Jiang SD, Jiang LS, Dai LY (2007) Changes in bone mass, bone structure, bone biomechanical properties, and bone metabolism after spinal cord injury: a 6-month longitudinal study in growing rats. Calcif Tissue Int 80:167–175
Duvall CL, Taylor WR, Weiss D, Guldberg RE (2004) Quantitative microcomputed tomography analysis of collateral vessel development after ischemic injury. Am J Physiol Heart Circ Physiol 287:H302–H310
Ding WG, Zhang ZM, Zhang YH, Jiang LS, Jiang SD, Dai LY (2010) Changes of substance P during fracture healing in ovariectomized mice. Regul Pept 159:28–34
Street J, Bao M, de Guzman L, Bunting S, Peale FV Jr, Ferrara N, Steinmetz H, Hoeffel J, Cleland JL, Daugherty A, van Bruggen N, Redmond HP, Carano RA, Filvaroff EH (2002) Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci USA 99:9656–9661
Nottage WM (1981) A review of long-bone fractures in patients with spinal cord injuries. Clin Orthop 155:65–70
Garland DE (1988) Clinical observations on fractures and heterotopic ossification in the spinal cord and traumatic brain injured populations. Clin Orthop Relat Res 233:86–101
Meyer RA Jr, Tsahakis PJ, Martin DF, Banks DM, Harrow ME, Kiebzak GM (2001) Age and ovariectomy impair both the normalization of mechanical properties and the accretion of mineral by the fracture callus in rats. J Orthop Res 19:428–435
Namkung-Matthai H, Appleyard R, Jansen J, Hao Lin J, Maastricht S, Swain M, Mason RS, Murrell GA, Diwan AD, Diamond T (2001) Osteoporosis influences the early period of fracture healing in a rat osteoporotic model. Bone 28:80–86
Lill CA, Hesseln J, Schlegel U, Eckhardt C, Goldhahn J, Schneider E (2003) Biomechanical evaluation of healing in a non-critical defect in a large animal model of osteoporosis. J Orthop Res 21:836–842
Maimoun L, Couret I, Micallef JP, Peruchon E, Mariano-Goulart D, Rossi M, Leroux JL, Ohanna F (2002) Use of bone biochemical markers with dual-energy x-ray absorptiometry for early determination of bone loss in persons with spinal cord injury. Metabolism 51:958–963
Pietschmann P, Pils P, Woloszczuk W, Maerk R, Lessan D, Stipicic J (1992) Increased serum osteocalcin levels in patients with paraplegia. Paraplegia 30:204–209
Roberts D, Lee W, Cuneo RC, Wittmann J, Ward G, Flatman R, McWhinney B, Hickman PE (1998) Longitudinal study of bone turnover after acute spinal cord injury. J Clin Endocrinol Metab 83:415–422
Duvall CL, Taylor WR, Weiss D, Wojtowicz AM, Guldberg RE (2007) Impaired angiogenesis, early callus formation, and late stage remodeling in fracture healing of osteopontin-deficient mice. J Bone Miner Res 22:286–297
Lu C, Marcucio R, Miclau T (2006) Assessing angiogenesis during fracture healing. Iowa Orthop J 26:17–26
Hisatome T, Yasunaga Y, Yanada S, Tabata Y, Ikada Y, Ochi M (2005) Neovascularization and bone regeneration by implantation of autologous bone marrow mononuclear cells. Biomaterials 26:4550–4556
Hausman MR, Schaffler MB, Majeska RJ (2001) Prevention of fracture healing in rats by an inhibitor of angiogenesis. Bone 29:560–564
Garcia-Sanz A, Rodriguez-Barbero A, Bentley MD, Ritman EL, Romero JC (1998) Three-dimensional microcomputed tomography of renal vasculature in rats. Hypertension 31(1 Pt 2):440–444
Guldberg RE, Ballock RT, Boyan BD, Duvall CL, Lin AS, Nagaraja S, Oest M, Phillips J, Porter BD, Robertson G, Taylor WR (2003) Analyzing bone, blood vessels, and biomaterials with microcomputed tomography. IEEE Eng Med Biol Mag 22:77–83
Bentley MD, Ortiz MC, Ritman EL, Romero JC (2002) The use of microcomputed tomography to study microvasculature in small rodents. Am J Physiol Regul Integr Comp Physiol 282:1267–1279
Keramaris NC, Calori GM, Nikolaou VS, Schemitsch EH, Giannoudis PV (2008) Fracture vascularity and bone healing: a systematic review of the role of VEGF. Injury 39(Suppl 2):S45–S57
Felsenberg D, Boonen S (2005) The bone quality framework: determinants of bone strength and their interrelationships, and implications for osteoporosis management. Clin Ther 27:1–11
Majumdar S, Kothari M, Augat P, Newitt DC, Link TM, Lin JC, Lang T, Lu Y, Genant HK (1998) High-resolution magnetic resonance imaging: three-dimensional trabecular bone architecture and biomechanical properties. Bone 22:445–454
Kleerekoper M, Villanueva AR, Stanciu J, Rao DS, Parfitt AM (1985) The role of three-dimensional trabecular microstructure in the pathogenesis of vertebral compression fractures. Calcif Tissue Int 37:594–597
Dempster DW (2000) The contribution of trabecular architecture to cancellous bone quality. J Bone Miner Res 15:20–23
Acknowledgments
This study was supported by the Science and Technology Commission of Shanghai Municipality (08JC1415800, 08411950100), Shanghai Rising-Star Program (08QA1405000), and Shanghai Educational Development Foundation (2008CG21).
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ding, WG., Jiang, SD., Zhang, YH. et al. Bone loss and impaired fracture healing in spinal cord injured mice. Osteoporos Int 22, 507–515 (2011). https://doi.org/10.1007/s00198-010-1256-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-010-1256-8