Abstract
Aims/hypothesis
Reactive oxygen species (ROS) generated during hyperglycaemia are implicated in the development of diabetic vascular complications. High glucose increases oxidative stress in endothelial cells and induces apoptosis. A major source of ROS in endothelial cells exposed to glucose is the NAD(P)H oxidase enzyme. Several studies demonstrated that C-peptide, the product of proinsulin cleavage within the pancreatic beta cells, displays anti-inflammatory effects in certain models of vascular dysfunction. However, the molecular mechanism underlying this effect is unclear. We hypothesised that C-peptide reduces glucose-induced ROS generation by decreasing NAD(P)H oxidase activation and prevents apoptosis
Methods
Human aortic endothelial cells (HAEC) were exposed to 25 mmol/l glucose in the presence or absence of C-peptide and tested for protein quantity and activity of caspase-3 and other apoptosis markers by ELISA, TUNEL and immunoblotting. Intracellular ROS were measured by flow cytometry using the ROS sensitive dye chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (CM-H2-DCDFA). NAD(P)H oxidase activation was assayed by lucigenin. Membrane and cytoplasmic levels of the NAD(P)H subunit ras-related C3 botulinum toxin substrate 1 (rho family, small GTP binding protein Rac1) (RAC-1) and its GTPase activity were studied by immunoblotting and ELISA. RAC-1 (also known as RAC1) gene expression was investigated by quantitative real-time PCR.
Results
C-peptide significantly decreased caspase-3 levels and activity and upregulated production of the anti-apoptotic factor B cell CLL/lymphoma 2 (BCL-2). Glucose-induced ROS production was quenched by C-peptide and this was associated with a decreased NAD(P)H oxidase activity and reduced RAC-1 membrane production and GTPase activity.
Conclusions/interpretation
In glucose-exposed endothelial cells, C-peptide acts as an endogenous antioxidant molecule by reducing RAC-1 translocation to membrane and NAD(P)H oxidase activation. By preventing oxidative stress, C-peptide protects endothelial cells from glucose-induced apoptosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Type 1 diabetes is a well-established risk factor for vascular disease [1]. Chronic elevations of blood glucose level (hyperglycaemia) and systemic low-grade inflammation contribute to the development of endothelial dysfunction, an early event in the pathogenesis of vascular disease in diabetes.
High glucose damages endothelial cells by increasing oxidative stress through generation of reactive oxygen species (ROS) [2–4], activation of the death protease caspase-3 [5], and inducing apoptosis [6, 7]. ROS are powerful cellular activators of the nuclear factor of κ light polypeptide gene enhancer in B cells 1 (NF-κB) pathway [8, 9], which regulates activation of a series of cytokine and adhesion molecule genes that results in the adhesion of leucocytes to endothelial cells and release of cytotoxic molecules. In human aortic endothelial cells (HAEC), activation of NF-κB accelerates apoptosis by downregulating production of B cell CLL/lymphoma 2 (BCL-2), an anti-apoptotic factor [10, 11].
High-glucose-induced ROS generation in endothelial cells mainly involves an NAD(P)H oxidase-dependent mechanism [12–15], which transfers electrons from NAD(P)H to molecular oxygen, producing O2 −. The NAD(P)H oxidase enzyme is composed of four functional components, the assembly of which requires the presence of the small GTP-binding protein ras-related C3 botulinum toxin substrate 1 (rho family, small GTP binding protein RAC1) (RAC-1) at the plasma membrane [16, 17]. In endothelial cells, RAC-1 controls low-intensity basal superoxide production as well as bursts of NAD(P)H oxidase activity [16], such as during exposure to high glucose [18, 19]. In type 1 diabetes, RAC-1-mediated ROS generation is considered an important pathophysiological pathway in the development of vascular complications [14, 20, 21]. In a recent report, it was shown that glucose-induced NAD(P)H oxidase activation, inflammatory responses and cardiovascular complications were attenuated in an animal model of Rac-1 (also known as Rac1) knockout [22]. This suggests that targeting inhibition of RAC-1 may represent an attractive therapeutic approach for reducing inflammatory-induced vascular damage in diabetes.
C-peptide, the cleavage product of the proinsulin molecule in the pancreatic beta cells, has been shown to exert insulin-independent biological effects on a number of cells, proving itself as a bioactive peptide with anti-inflammatory properties [23]. As type 1 diabetes patients typically lack physiological levels of insulin and C-peptide, this is considered an important factor in the pathophysiology of diabetic complications [24–26]. C-peptide has been shown to improve endothelial dysfunction and systemic inflammation in several in vivo and in vitro models of inflammation-mediated vascular injury by reducing expression of genes encoding endothelial cell adhesion molecules, inflammatory cytokine production and adherence and transmigration of leucocytes [27–30]. Although the exact mechanism(s) underlying the anti-inflammatory activity of C-peptide is not known, there is evidence that C-peptide affects NF-κB activation [29, 31]. However, which NF-κB-dependent upstream signalling event is affected by C-peptide in endothelial cells is not clear.
We hypothesised that C-peptide acts as an antioxidant molecule by reducing high-glucose-induced ROS generation in endothelial cells. Therefore, in this study, we examined the effect of C-peptide on high-glucose-induced ROS generation as the mechanism underlying its beneficial effects on endothelial cell dysfunction and apoptosis. We focused on the effect of C-peptide on the RAC-1 pathway of ROS generation, which is recognised as the major pathway of ROS production in endothelial cells during diabetes.
Methods
Cells
HAEC were obtained from Lonza (Lonza, Walkersville, MD, USA) and maintained in T-75 cm2 flasks (Corning, New York, NY, USA) at 37°C, 95% air and 5% CO2 in EBM-2 (Lonza) supplemented with endothelial growth medium 2 (EGM-2) kit SingleQuots (Lonza). EBM-2 contains 5.5 mmol/l glucose, which is considered the normal glucose level required for HAEC survival. In all experiments, the high-glucose medium was EBM-2 containing 25 mmol/l glucose (Sigma-Aldrich, St Louis, MO, USA). HAEC were used when they reached 90% confluency and up to the sixth passage.
Treatment conditions
HAEC were exposed to regular EBM-2, high-glucose medium, or high-glucose medium with either human C-peptide (Phoenix Pharmaceuticals, Burlingame, CA, USA) or scrambled human C-peptide (Sigma-Genosys, The Woodlands, TX, USA) (10 nmol/l) (purity ≥95%) for a time period ranging from 30 min to 48 h, as specified in each experiment. In experiments to detect RAC-1 mRNA and protein production, and RAC-1 GTPase and NAD(P)H oxidase activities, human EGF was removed from the media to avoid aspecific RAC activation. To study TNF-α-mediated apoptosis, HAEC were pre-treated for 24 h with C-peptide (10 nmol/l) and then exposed to TNF-α (20 ng/ml) (R&D Systems, Minneapolis, MN, USA) for 24 h. All experiments were performed at 37°C, 95% air and 5% CO2. The dose of 10 nmol/l C-peptide was selected because it showed significant anti-apoptotic effects in dose–response experiments. Unless otherwise indicated, for each assay a minimum of three independent experiments were run in which each condition was tested in triplicate.
Detection of apoptosis
HAEC were seeded in 96 well plates and the next day treated as specified above for 48 h. Apoptosis was detected using the Cell Death Detection ELISAPLUS kit (Roche Diagnostics, Mannheim, Germany). Results were expressed as absorbance raw data (mean±SD). Apoptosis was also detected with a TUNEL assay using the In Situ Cell Death Detection Kit, Fluorescein (Roche Diagnostics) according to the manufacturer’s instructions. This assay was performed on HAEC seeded on MatTek plates (MatTek, Ashland, MA, USA) and exposed for 96 h to the treatment conditions as above. The label incorporated at the damaged sites of DNA was visualised by confocal fluorescent microscopy (Olympus Fluoview PV1000, Center Valley, PA, USA) at ×40 magnification.
Immunoblotting for BAX, BCL-2, cleaved caspase-3 and RAC-1
For BCL2-associated X protein (BAX), BCL-2 and cleaved caspase-3 protein detection, HAEC were exposed overnight to the treatment conditions as above. For RAC-1 detection, HAEC were serum starved overnight before exposing to the treatment conditions for 30 min. Cytosolic and membrane proteins were extracted using Qproteame Cell Compartment kit (Qiagen, Valencia, CA, USA) and protein content was measured using a bicinchoninic acid assay kit (Pierce Biotechnology, Thermo Scientific, Rockford, IL, USA). Aliquots of protein extracts (30 μg) were subject to immunoblot analysis using rabbit polyclonal anti-RAC-1 (1:1000), anti-cleaved caspase-3 (1:500), anti-BCL-2 antibodies (all from Cell Signaling Technology, Danvers, MA, USA) and mouse monoclonal anti-β-actin antibody (1:10,000; Sigma). A rabbit polyclonal antibody anti-BAX (1:500) (Millipore, Billerica, MA, USA) was used to detect BAX protein levels. Densitometry was performed with UN-SCAN-IT gel software (Silk Scientific, Orem, UT, USA).
Assays of caspase-3 enzyme activity
HAEC were maintained in 96 well plates and exposed to treatment conditions as above overnight. Caspase-3 activity was assessed in cytoplasmic cell lysates using the Caspase-3 Activity Assay Kit following manufacturer’s instructions (Calbiochem, EMD Chemicals, Gibbstown, NJ, USA). Results were expressed as caspase-3 activity fold induction vs normal glucose condition (mean±SD).
Determination of intracellular ROS
HAEC (50,000/well) were seeded in six-well plates and treated overnight as specified above. Intracellular hydrogen peroxide (H2O2) production was monitored over time by flow cytometry using chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (CM-H2-DCFDA; 10μmol/l; Molecular Probes, Invitrogen), as specified in the electronic supplementary material (ESM). At least four experiments were run in which each condition was tested in duplicate. Results are expressed as mean fluorescence of CM-H2-DCFDA.
NAD(P)H oxidase activity detection
Glucose-induced NAD(P)H oxidase activity was measured in live HAEC exposed to the different treatment conditions for 30 min using lucigenin-derived chemiluminescence, as described by Mustapha et al. [32]. For a more detailed description of methods, see the ESM. Three experiments were performed in which each condition was tested in quadruplicate. Results were expressed as percentage (mean±SD) of NAD(P)H oxidase activity.
Measure of RAC-1 mRNA expression by quantitative real-time PCR
HAEC were serum starved overnight and exposed to treatment conditions for 30 min. Total RNA was isolated using RNAqueous-4PCR kit (Ambion, Austin, TX, USA) and quantified by spectrophotometry. RNA, 1 μg, was reverse transcribed to cDNA (5 min at 65°C, 50 min at 50°C and 5 min at 85°C) using oligo(dT) primers (Invitrogen, Carlsbad, CA, USA) and quantitative real-time PCR was performed to amplify RAC-1 and the housekee** gene human GAPDH [33]. Sequences of the oligonucleotides used to amplify these genes are reported in the ESM. RAC-1 data were normalised using the GAPDH housekee** gene and results were expressed as fold induction vs normal glucose conditions (mean±SD of three independent experiments).
Assessment of RAC-1 GTPase activity
HAEC were serum starved overnight and exposed to treatment conditions for 30 min. RAC GTPase activity was measured in 10 μg of cell lysates using the RAC G-LISA Activation Assay kit following the manufacturer’s instructions (Cytoskeleton, Denver, CO, USA). At least four experiments were run in which each condition was tested in duplicate. Results are expressed as fold induction of GTPase activity (mean±SD) compared with normal glucose conditions.
Statistical analysis
ANOVA followed by Dunnett’s post hoc test was used to assess differences between the different conditions using GraphPad Prism 4 (GraphPad Software, San Diego, CA, USA). Values of p < 0.05 were considered to be statistically significant.
Results
C-peptide decreases high-glucose-induced apoptosis of HAEC
Exposure of HAEC to high glucose for 48 h significantly increased apoptosis as compared with normal glucose (Fig. 1a,b; p < 0.01). The addition of 10 nmol/l C-peptide decreased glucose-induced apoptosis of HAEC (Fig. 1a,b; p < 0.01 vs high glucose). This effect corresponded to a 25% reduction of apoptosis by C-peptide as compared with high glucose. Higher concentrations of C-peptide (20 and 50 nmol/l) did not have any significant effects on glucose-induced apoptosis (Fig. 1a). In Fig. 1b, addition of scrambled C-peptide (10 nmol/l) to the high-glucose medium did not cause any significant effects on HAEC apoptosis, suggesting that the beneficial effect was specific to C-peptide.
C-peptide decreases glucose-induced apoptosis of HAEC. a HAEC were exposed to normal glucose, or to high glucose (25 mmol/l) alone or in the presence of a range of C-peptide concentrations for 48 h and tested for cytoplasmic histone-associated DNA fragments by using the Cell Death Detection ELISAPLUS. b HAEC were exposed to normal glucose, or to high glucose alone or with either C-peptide or scrambled C-peptide (10 nmol/l) for 48 h and tested for apoptosis as in (a). A significant increase in apoptosis was found in high-glucose-exposed HAEC compared with cells exposed to normal glucose (**p < 0.01). C-peptide at 10 nmol/l, but not scrambled C-peptide, decreased apoptosis (†† p < 0.01 vs high glucose). Higher concentrations of CP were not effective. Values are mean±SD of three different experiments in which each condition was tested in triplicate. CP, C-peptide; HG, high glucose; NG, normal glucose; Scr., scrambled
Glucose-induced endothelial apoptosis was also evaluated by TUNEL assay under a confocal fluorescent microscopy (Fig. 2). As compared with normal glucose, HAEC exposed to high glucose demonstrated a significant induction of apoptosis that was reduced by C-peptide (10 nmol/l). Scrambled C-peptide was without any significant effect as compared with high glucose alone (Fig. 2).
TUNEL assay in glucose-exposed HAEC cultures: (a) normal glucose; (b) high glucose; (c) high glucose+C-peptide; and (d) high glucose+scrambled C-peptide. TUNEL staining shows an increase in apoptosis in HAEC (in green) exposed to high glucose compared with cells exposed to normal glucose. C-peptide reduced the number of TUNEL+ cells compared with high glucose alone. Scrambled C-peptide had no apparent effect. Shown are representative images of three independent experiments (×40 magnification). CP, C-peptide; scr., scrambled
C-peptide decreases high-glucose-induced caspase-3 production and activity in HAEC
One crucial mediator of apoptosis is the activated caspase-3 protease, which catalyses the cleavage of many key cellular proteins. We evaluated endogenous levels of the large fragment (17/19 kDa) of activated (cleaved) caspase-3 by western blotting in cytoplasmic lysates from HAEC exposed to high glucose overnight. As shown in Fig. 3a, production of activated caspase-3 doubled in lysates from HAEC exposed to high glucose compared with normal glucose (p < 0.05). Addition of C-peptide reduced caspase-3 levels to those detected in normal glucose (p < 0.05 vs high glucose), a result that was not observed with scrambled C-peptide (Fig. 3a).
C-peptide decreases cleaved caspase-3 protein levels and activity in HAEC exposed to high glucose. HAEC were cultured in normal glucose or high glucose (25 mmol/l) in the presence or absence of either C-peptide or scrambled C-peptide (10 nmol/l) overnight. a Cytoplasmic extracts were subjected to western blotting to detect cleaved caspase-3. Densitometric quantification of the bands showed that in cells exposed to high glucose there was a twofold increase in caspase-3 protein levels compared with cells exposed to normal glucose (*p < 0.05). C-peptide significantly decreased caspase-3 levels († p < 0.05 vs high glucose). b A 1.5-fold increase in caspase-3 activity was measured in high-glucose-exposed HAEC compared with those exposed to normal glucose (**p < 0.01). C-peptide treatment overnight reduced caspase-3 activity to levels detected in normal glucose (†† p < 0.01 vs high glucose). Results are expressed as mean±SD (n = 3). CP, C-peptide; HG, high glucose; NG, normal glucose; Scr., scrambled
Caspase-3 activity was evaluated in cytoplasmic lysates from high-glucose-exposed HAEC by ELISA. Exposure to high glucose overnight significantly increased caspase-3 activity 1.5-fold compared with normal glucose (Fig. 3b; p < 0.01). Addition of C-peptide, significantly reduced caspase-3 activity to levels detected in normal glucose (p < 0.01 vs high glucose alone), while scrambled C-peptide showed no significant effects (Fig. 3b).
C-peptide increases production of the anti-apoptotic factor BCL-2 in high glucose-treated HAEC
Analysis of the product of the survival gene BCL-2 by western blotting showed that overnight exposure to high glucose decreased BCL-2 production by 50% compared with levels detected in normal glucose (Fig. 4a, p < 0.05). Addition of C-peptide increased BCL-2 production to levels detected under normal glucose (Fig. 4a, p < 0.05 vs high glucose). C-peptide did not change the levels of the pro-apoptotic molecule BAX in glucose-exposed HAEC compared with cells exposed to high glucose (Fig. 4b).
C-peptide increases BCL-2 production in HAEC exposed to high glucose. a Representative immunoblot of BCL-2 and β-actin in extracts from HAEC incubated overnight in: normal glucose; high glucose (25 mmol/l); or high glucose+10 nmol/l C-peptide. Densitometric quantification of the bands showed significantly lower BCL-2 levels in high-glucose-exposed cells compared with those exposed to normal glucose (*p < 0.05). Addition of C-peptide triggered an increase in BCL-2 levels († p < 0.05 vs high glucose). Results are expressed as mean±SD (n = 3). b Representative immunoblot of the pro-apoptotic molecule BAX in extracts from HAEC incubated overnight in: normal glucose; high glucose (25 mmol/l); or high glucose+C-peptide or scrambled C-peptide (10 nmol/l). C-peptide did not change the production of BAX in glucose-exposed HAEC. CP, C-peptide; HG, high glucose; NG, normal glucose; Scr., scrambled
C-peptide reduces TNF-α-mediated apoptosis of HAEC
As an additional model of apoptosis, we investigated the one mediated by the inflammatory cytokine TNF-α, which plays an important role in the development of diabetic complications [34]. Exposure of HAEC to TNF-α significantly increased apoptosis as compared with normal glucose (Fig. 5a; p < 0.01). Addition of C-peptide significantly reduced apoptosis as compared with TNF-α alone (Fig. 5a; p < 0.01).
C-peptide antagonises TNF-α-mediated apoptosis of HAEC. HAEC were exposed to normal glucose with or without TNF-α (20 ng/ml) in the presence or absence of C-peptide (10 nmol/l) for 24 h. a Changes in cytoplasmic histone-associated DNA fragments detected using the Cell Death Detection ELISAPLUS. A significant increase in apoptosis was observed in TNF-α-exposed HAEC compared with those exposed to normal glucose alone (**p < 0.01). Addition of C-peptide significantly reduced TNF-α-induced apoptosis as compared with HAEC exposed to TNF-α alone (†† p < 0.01). Results are expressed as mean±SD (n = 3). b Representative image of immunoblot showing cleaved caspase-3 levels in HAEC exposed to the different conditions as above. While endogenous cleaved caspase-3 levels increased after exposure to TNF-α compared with medium alone, addition of C-peptide decreased caspase-3 levels. BCL-2 protein levels in HAEC decreased after exposure to TNF-α. Addition of C-peptide increased BCL-2 production to levels detected with normal glucose
Caspase-3 levels were higher in lysates from HAEC treated with TNF-α compared with normal glucose (Fig. 5b). C-peptide reduced activated caspase-3 to levels observed under normal glucose (Fig. 5b). Analysis of BCL-2 production by western blotting showed that while TNF-α decreased BCL-2 levels compared with normal glucose, addition of C-peptide reversed this condition by increasing BCL-2 production to levels detected under normal glucose (Fig. 5b).
C-peptide reduces high glucose-induced ROS production in HAEC
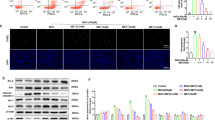
Figure 6a shows results from a representative experiment in which intracellular ROS generation in HAEC was assessed over time by flow cytometry analysis of the ROS sensitive dye CM-H2-DCFDA in the gated cells. We found that at time 0, after overnight incubation with normal glucose (blue), HAEC showed a basal level of ROS, which continued to increase over time up to 3 h. ROS production was higher in HAEC exposed to high glucose (red) (p < 0.05). In HAEC treated with high glucose and C-peptide (green), ROS generation was always lower than in cells exposed to high glucose alone (red) and this difference reached statistical significance at the 3 h and 4 h time points (p < 0.05) (Fig. 6a). When scrambled C-peptide was added to high glucose (grey), no significant decrease in DCFDA fluorescence was detected in HAEC as compared with cells exposed to high glucose alone (red) (Fig. 6a). Figure 6b shows representative histograms of flow cytometry analysis of CM-H2-DCFDA fluorescence in HAEC under the different treatment conditions at the 3 h time point.
C-peptide reduces high-glucose-induced ROS generation in HAEC. Intracellular ROS accumulation in HAEC exposed overnight to normal glucose (blue); high glucose (25 mmol/l; red); high glucose+C-peptide (green) or high glucose+scrambled C-peptide (10 nmol/l; grey). The following day, the ROS-sensitive dye CM-H2-DCFDA (10 μmol/l) was added for 30 min in an incubator after which cells were run on a flow cytometer (time 0) and every hour for a total of 5 h. a Representative time course analysis of ROS generation expressed as mean fluorescence intensity of CM-H2-DCFDA. At time 0, after overnight incubation, HAEC in normal glucose showed a basal level of ROS, which continued to increase over time up to 3 h. HAEC in high glucose produced higher ROS (*p < 0.05 vs normal glucose). C-peptide lowered glucose-induced ROS production at all time points, but reached significance at 3 h and 4 h († p < 0.05 vs high glucose). Scrambled C-peptide had no significant effect compared with high glucose alone. b–e Representative histograms of flow cytometry analysis of ROS detection at 3 h: (b) normal glucose; (c) high glucose; (d) high glucose+C-peptide; (e) high glucose+scrambled C-peptide. Each histogram shows fluorescence intensity (on the x-axis) and number of events (counts) on the y-axis. The peak on the left represents negative cells, while the peak on the right represents cells that positively stain with CM-H2-DCFDA. In the histogram of cells exposed to high glucose (c), the number of CM-H2-DCFDA positive cells increased as compared with normal glucose (b). When C-peptide was added to the high glucose medium (d), the number of CM-H2-DCFDA-positive cells decreased, while scrambled C-peptide was without any significant effects (e)
C-peptide reduces NAD(P)H oxidase activity in high glucose-exposed HAEC
Exposure of HAEC to high glucose for 30 min increased NAD(P)H oxidase activity of 50% as compared with normal glucose (p = 0.01). C-peptide added to the medium for 30 min significantly downregulated NAD(P)H oxidase activity as compared with high glucose alone (p < 0.01), while scrambled C-peptide did not have any significant effect (Fig. 7). As expected, the pharmacological NAD(P)H oxidase inhibitors diphenyliodonium (DPI) and apocynin significantly abolished high-glucose-induced NAD(P)H oxidase activation (p < 0.01 and p = 0.01, respectively).
C-peptide reduces high-glucose-induced NAD(P)H oxidase activation in HAEC. HAEC were exposed to: normal glucose; or high glucose (25 mmol/l) in the presence or absence of either C-peptide or scrambled C-peptide (10 nmol/l) as a control. After 30 min, high glucose increased NAD(P)H oxidase activity compared with normal glucose (**p = 0.01). C-peptide, but not scrambled C-peptide, reduced high-glucose-induced NAD(P)H oxidase activity (†† p < 0.01 vs high glucose). Pre-treatment with the pharmacological inhibitors apocynin (10 μmol/l) and DPI (100 μmol/l) drastically reduced high-glucose-induced NAD(P)H oxidase activity in HAEC (‡‡ p = 0.01 DPI vs high glucose; ***p < 0.001 apocynin vs high glucose). Results are expressed as percentage (mean±SD) of NAD(P)H oxidase activity in three independent experiments. CP, C-peptide; HG, high glucose; NG, normal glucose; Scr., scrambled
C-peptide reduces high-glucose-induced production of RAC-1 at the plasma membrane and its GTPase activity
Assembly of the four functional components to form active NAD(P)H oxidase requires the presence of the small GTP-binding protein RAC-1. Therefore, we investigated RAC-1 protein levels in the cytoplasm and plasma membrane of high-glucose-exposed HAEC by western blotting. Exposure of HAEC to high glucose for 30 min triggered translocation of RAC-1 from the cytoplasm to the plasma membrane as compared with exposure to regular medium (Fig. 8a; p < 0.05). C-peptide significantly reduced RAC-1 translocation from the cytoplasm to the membrane (p < 0.05 vs high glucose; Fig. 8a).
Effect of C-peptide on RAC-1 translocation and GTPase activity in HAEC exposed to high glucose. HAEC were serum starved overnight and exposed to normal glucose or high glucose (25 mmol/l) in the presence or absence of either C-peptide or scrambled C-peptide (10 nmol/l) for 30 min at 37°C. a Western blot of cytoplasmic (RAC-1-c) and membrane (RAC-1-m) in glucose-exposed HAEC. Bar graphs show densitometric quantification of RAC-1-m. High glucose induced increased levels of RAC-1 in the plasma membrane (*p < 0.05 compared with normal glucose). C-peptide treatment decreased translocation of RAC-1 from the cytoplasm to the membrane († p < 0.05 vs high glucose). b Cell lysates were subjected to the G-LISA assay to detect RAC GTPase activity. High glucose increased GTPase activity in HAEC after 30 min compared with normal glucose (**p < 0.01). Addition of C-peptide decreased GTPase activity to levels measured in normal glucose (†† p < 0.01 vs high glucose). c Quantitative real-time PCR analysis of RAC-1 mRNA gene expression in HAEC after 30 min exposure to the different treatment conditions as above. No significant differences were found in RAC-1 mRNA gene expression in cells exposed to the various treatments. Results are expressed as (mean±SD) of three independent experiments
RAC is a member of the Rho family of small GTPases that undergo regulatory control by alternating between binding GTP for activation and hydrolysis to GDP for inactivation. We investigated whether intrinsic RAC-1 GTPase activity was affected by C-peptide. In Fig. 8b, HAEC exposed to high glucose for 30 min significantly increased GTPase activity by 50% compared with cells exposed to regular glucose medium (p < 0.01). When C-peptide was added to high glucose, it decreased the GTPase activity of 25% compared with high glucose alone (p < 0.01). Scrambled C-peptide did not significantly affect GTPase activation.
C-peptide does not affect RAC-1 mRNA gene expression in high-glucose-exposed HAEC
We tested whether C-peptide treatment for 30 min had any effects on RAC-1 mRNA gene expression in high-glucose-exposed HAEC. As shown in Fig. 8c, we did not find any significant differences in RAC-1 mRNA expression in HAEC exposed to the different conditions tested.
Discussion
It has been reported that high glucose increases ROS generation in HAEC [2–4] and triggers apoptosis [6, 7, 10]. ROS production causes apoptotic cell death in endothelial cells [5, 7, 10] and plays an important role in the development of diabetic vascular complications [2, 4]. Indeed, it has been shown that antioxidant agents rescue hyperglycaemia-induced endothelial dysfunction and reduce the risk of coronary heart disease [35, 36]. In this study, we have demonstrated that C-peptide reduced high-glucose-induced apoptosis and quenched glucose-induced oxidative stress in endothelial cells, an effect conveyed through the inhibition of NAD(P)H oxidase. Furthermore, we demonstrated that the effect of C-peptide on glucose-induced NAD(P)H-oxidase-derived ROS production is mediated by an inhibition of RAC-1 translocation, a crucial component of NAD(P)H oxidase.
C-peptide is the cleavage product of the proinsulin molecule generated in the pancreatic beta cells of healthy individuals and co-released together with insulin in the peripheral circulation in response to elevation of blood glucose levels. In individuals with type 1 diabetes, both insulin and C-peptide are missing because of autoimmune destruction of the pancreatic beta cells. As a consequence, individuals with type 1 diabetes have severely reduced levels or absence of C-peptide; this is considered an important factor in the pathophysiology of diabetic complications. In fact, people with type 1 diabetes who retain a low but detectable level of C-peptide are less prone to develop microvascular complications of the eyes, kidneys and peripheral nerves [24–26]. Moreover, pancreas or islet transplantation, with restoration of endogenous insulin and C-peptide secretion, is known to be accompanied by improvement of diabetes-induced abnormalities of nerve function, endothelial function and both structural and functional changes of the kidneys [37, 38]. C-peptide has been shown to display anti-inflammatory activity on endothelial cells exposed to a variety of damaging insults and to be beneficial in endothelial dysfunction during type 1 diabetes [39]. In this regard, pretreatment with C-peptide to rats injected with the inflammatory agents thrombin or \( {N^{\omega }} \)-nitro-l-arginine methyl ester (l-NAME), which cause acute endothelial dysfunction, resulted in reduced expression of intercellular cell adhesion molecule (ICAM)-1 and P-selectin on the mesenteric microvascular endothelium [28]. As a consequence, the number of rolling, adhering and transmigrated leucocytes also decreased upon C-peptide administration to the animals. In another model of vascular injury, systemic administration of C-peptide decreased polymorphonuclear leucocyte infiltration in isolated rat hearts following ischaemia–reperfusion injury and restored cardiac contractile function and postreperfusion coronary heart flow [27]. Our group has reported on the anti-inflammatory activity of C-peptide in high-glucose endothelial dysfunction, when C-peptide decreased vascular cell adhesion molecule 1 (VCAM1) mRNA expression and protein levels, and reduced secretion of IL-8 and monocyte chemoattractant factor (MCP)-1 by HAEC to the basal levels measured under normal glucose concentrations [29].
In this current study, we demonstrated that C-peptide reduced glucose-induced apoptosis of HAEC. Activation of caspase-3 is a central component of the proteolytic cascade in glucose-induced apoptosis of human endothelial cells [5]. In our model, we found that overnight exposure to high glucose increased levels and activity of activated caspase-3, which was reduced by addition of C-peptide to HAEC in vitro. Moreover, in agreement with Bugliani et al., who studied human pancreatic beta cells, levels of the anti-apoptotic molecule BCL-2, but not of the pro-apoptotic molecule BAX, were upregulated by C-peptide compared with high glucose alone in HAEC [40]. BAX belongs to the BCL-2 protein family of apoptosis-regulator gene products that may function as apoptotic activators (BAX, BCL2-antagonist/killer 1 [BAK], BCL2-associated agonist of cell death [BAD], and others) or facilitating cell survival (BCL-2, BCL2-like 1 [BCL-XL], BCL2-like 2 [BCL-W], and others) [41]. Although the protective effect of C-peptide in endothelial cell apoptosis is reported here for the first time, the anti-apoptotic effect of C-peptide has been already described in different cellular models. Using human neuroblastoma SH-SY5Y cells, Li et al. found that C-peptide reduced high-glucose-induced apoptosis by promoting the expression of BCL-2 (also known as BCL2) [42]. In addition, in the BB/Wor rat model of spontaneous type 1 diabetes, C-peptide decreased hippocampal cell apoptosis, which was accompanied by lowered caspase-3 activation [43]. Finally, in this paper we show that C-peptide reduced TNF-α-induced HAEC apoptosis, decreased expression of caspase-3 and upregulated BCL-2. A similar result was reported by Al-Rasheed et al. in opossum kidney proximal tubular cells [34]. Taken together, these findings support the view that C-peptide prevents cellular apoptosis mediated by different inflammatory stimuli.
Although it has been shown that C-peptide acts via Gαi possibly via a G-protein-coupled receptor to protect against TNF-α-induced apoptosis in kidney proximal tubular cells [34], the intracellular mechanisms of C-peptide-mediated anti-apoptotic effects in endothelial cells are not well understood. In high-glucose-exposed endothelial cells, cellular apoptosis involves oxidative-stress-triggered activation of the NF-κB pathway [7, 10] which, in turn, suppresses BCL-2 levels and activates caspase-3 activity [10, 44]. We have previously observed that C-peptide interferes with glucose-induced nuclear translocation of the NF-κB p65/p50 subunits in HAEC, and reduces endothelial dysfunction [29]. An effect of C-peptide on NF-κB and consequent decreased inflammatory cytokine production has also been reported in the brain of diabetic BB/Wor rats and found to be associated with reduced neuronal apoptosis [31, 45, 46]. Here, we add significant pieces of information, by showing that C-peptide decreases intracellular ROS generation, a crucial upstream signalling event in the NF-κB pathway. In our model, ROS generation in HAEC was measured after overnight incubation with high glucose. C-peptide treatment quenched high-glucose-induced ROS production to bring levels closer to those detected in normal glucose at all time points, reaching statistical significance at 3 h, thus suggesting that C-peptide exerts its beneficial effects on glucose-exposed endothelial cells over time. Our results are in apparent contrast with those from Stevens et al. [47], who reported no changes in antioxidant enzymatic activity in sciatic nerve homogenates from diabetic BB/Wor rats who were administered C-peptide for 2 months as compared with animals who did not receive C-peptide, although amelioration of endoneural nerve blood flow was found.
A possible explanation for these contrasting results could lie in the different methods used to detect oxidative stress and to the different experimental conditions employed in the two studies. While Stevens et al. determined levels of antioxidant enzymes in homogenates of rat sciatic nerves, we directly measured intracellular ROS production in live cultured HAEC after short exposure to high glucose. Thus, C-peptide might have different effects in different tissues under different experimental conditions. For example, one could speculate that timing of cellular exposure to C-peptide might be important as the most meaningful beneficial effects of C-peptide on oxidative stress are rapid, thus suggesting that C-peptide acts at the very early stages of glucose-induced vascular dysfunction. Furthermore, it might be that nerve cells and endothelial cells have different basal activities and mRNA levels of antioxidant enzymes so that one cell is more susceptible to oxidative stress than another. Antioxidant enzymatic activity of C-peptide-treated cells was not investigated in our study. In addition, the exact antioxidant enzymes that are induced by high glucose in HAEC and whether C-peptide is able to affect their mRNA levels or activities are not known.
We showed that C-peptide inhibits glucose-induced NAD(P)H oxidase activation, which is the major source of ROS in endothelial cells. This multi-component enzyme includes a membrane-bound cytochrome b 558 , comprised of p22phox and gp91phox subunits, and the cytosolic adapter proteins p47phox and p67phox, which are recruited to the cytochrome during stimulation to form a catalytically active oxidase [16, 17]. Recruitment of p47phox and p67phox to the plasma membrane requires the presence of RAC-1, a member of the rho family of small GTP-binding proteins that complex with the cytosolic proteins to regulate NAD(P)H oxidase activity. In this study, we report that glucose-induced RAC-1 protein levels at the plasma membrane of HAEC were reduced by 30 min treatment with C-peptide in vitro. Moreover, glucose-induced RAC GTPase activity was also reduced by C-peptide in HAEC. All together, these findings demonstrate that C-peptide decreases ROS generation by affecting RAC-1-dependent NAD(P)H oxidase activation in glucose-exposed HAEC. Thus, based on these findings we suggest that C-peptide in healthy individuals may represent an endogenous molecule with antioxidant properties that, once secreted in the bloodstream, protects the vascular endothelium from the damaging effects of hyperglycaemia-induced oxidative stress. An effect of C-peptide on preserving endothelial function by affecting indices of oxidative stress in individuals with diabetes was reported a few years ago by Manzella et al. [48].
How exactly C-peptide interferes with RAC-1-mediated NAD(P)H generation of ROS is not known. Based on our data, we support the hypothesis that C-peptide may interfere with translocation of RAC-1 from the cytoplasm to the membrane. In fact, membrane levels of RAC-1 and its GTPase activity were significantly reduced in C-peptide-treated endothelial cells. In our model, no effect by C-peptide on RAC-1 mRNA gene expression was detected after 30 min exposure. Thus, we conclude that C-peptide may have an effect on post-translational modifications (i.e. isoprenylation) of RAC-1 that are required for translocation to the plasma membrane upon activation [49]. In addition, C-peptide may also affect translocation of the other NAD(P)H cytoplasmic subunits p67phox and/or p47phox which, when bound to RAC-1, can migrate from the cytoplasm to plasma membrane where activation of the cytochrome occurs. Further studies are necessary to investigate these theories.
We conclude that C-peptide prevents apoptosis in high-glucose-exposed HAEC by reducing oxidative stress. We have identified the RAC-1 pathway as a potential intracellular target of C-peptide in reducing ROS generation and apoptosis.
Abbreviations
- BAX:
-
BCL-2-associated X protein
- BCL-2:
-
B cell CLL/lymphoma 2
- CM-H2-DCDFA:
-
Chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate
- DPI:
-
Diphenyliodonium
- HAEC:
-
Human aortic endothelial cells
- NF-κB:
-
Nuclear factor of κ light polypeptide gene enhancer in B cells 1
- RAC-1:
-
Ras-related C3 botulinum toxin substrate 1 (rho family, small GTP binding protein RAC1)
- ROS:
-
Reactive oxygen species
References
Libby P, Nathan DM, Abraham K et al (2005) Report of the National Heart, Lung, and Blood Institute-National Institute of Diabetes and Digestive and Kidney Diseases Working Group on Cardiovascular Complications of Type 1 Diabetes Mellitus. Circulation 111:3489–3493
Baynes JW (1991) Role of oxidative stress in development of complications in diabetes. Diabetes 40:405–412
Srinivasan S, Yeh M, Danziger EC et al (2003) Glucose regulates monocyte adhesion through endothelial production of interleukin-8. Circ Res 92:371–377
Li J, Shah AM (2004) Endothelial cell superoxide generation: regulation and relevance for cardiovascular pathophysiology. Am J Physiol Regulatory Integrative Comp Physiol 287:R1014–R1030
Ho FM, Liu SH, Liau CS, Huang PJ, Lian-Shiau SH (2000) High glucose-induced apoptosis in human endothelial cells is mediated by sequential activations of c-Jun NH2-terminal kinase and caspase-3. Circulation 101:2618–2624
Baumgartner-Parzer SM, Wagner L, Pettermann M, Grillari J, Gessi A, Waldhausi W (1995) High glucose-triggered apoptosis in cultured endothelial cells. Diabetes 44:1323–1327
Du XL, Sui GZ, Stockklauser-Färber K et al (1998) Introduction of apoptosis by high proinsulin and glucose in cultured human umbilical vein endothelial cells is mediated by reactive oxygen species. Diabetologia 41:249–256
Barchowsky A, Munro SR, Morana SJ, Vincenti MP, Treadwell M (1995) Oxidant-sensitive and phosphorylation-dependent activation of NF-kappa B and AP-1 in endothelial cells. Am J Physiol 269:829–836
Janssen-Heininger YM, Poynter ME, Baeuerle PA (2000) Recent advances towards understanding redox mechanims in the activation of nuclear factor kappaB. Free Radic Biol Med 28:1317–1327
Aoki M, Nata T, Morishita R et al (2001) Endothelial apoptosis induced by oxidative stress through activation of NF-kappa B: antiapoptotic effect of antioxidant agents on endothelial cells. Hypertension 38:48–55
Matsushita H, Morishita R, Nata T et al (2000) Hypoxia-induced endothelial apoptosis through nuclear factor-kB (NF-kB)-mediated bcl-2 suppression. Circ Res 86:974–981
Griendling KK, Sorescu D, Ushio-Fukai M (2000) NAD(P)H oxidase: Role in cardiovascular biology and disease. Circ Res 86:494–501
Wautier JL, Schmidt AM (2004) Protein glycation: a firm link to endothelial cell dysfunction. Circ Res 95:233–238
Gao L, Mann GE (2009) Vascular NAD(P)H oxidase activation in diabetes: a double-edged sword in redox signaling. Cardiovasc Res 82:9–20
Ray R, Shah AM (2005) NADPH oxidase and endothelial cell function. Clin Sci 109:217–226
Gregg D, Rauscher FM, Goldschmidt-Clermont PJ (2003) Rac regulates cardiovascular superoxide through diverse molecular interactions: more than a binary GTP switch. Am J Physiol Cell Physiol 285:C723–C734
Hordijk PL (2006) Regulation of NADPH oxidases. The role of Rac proteins. Circ Res 98:453–462
Schmidt AM, Yan SD, Stern DM (1995) The dark side of glucose. Nat Med 1:1002–1004
Gallo A, Ceolotto G, Pinton P et al (2005) Metformin prevents glucose-induced protein kinase C-beta2 activation in human umbilical vein endothelial cells through an antioxidant mechanism. Diabetes 54:1123–1131
Martin-Gallan P, Carrascosa A, Gussinyen M, Dominguez C (2003) Biomarkers of diabetes-associated stress and antioxidant status in young diabetic patients with or without subclinical complications. Free Radic Biol Med 15:1563–1574
Harrison D, Grindling KK, Landmesser U, Horning B, Drexler H (2003) Role of oxidative stress in atherosclerosis. Am J Cardiol 91:7A–11A
Li J, Zhu H, Shen E, Wan L, Arnold JMO, Peng T (2010) Deficiency of Rac1 blocks NADPH oxidase activation, inhibits endoplasmic reticulum stress, and reduces myocardial remodeling in a mouse model of type 1 diabetes. Diabetes 59:2033–2042
Luppi P, Cifarelli V, Wahren J (2011) C-peptide and long-term complication of diabetes. Pediatr Diabetes 12:276–292
Sjöberg S, Gunnarsson R, Gjötterberg M, Lefvert AK, Persson A, Ostman J (1987) Residual insulin production, glycaemic control and prevalence of microvascular lesions and polyneuropathy in long-term type 1 (insulin-dependent) diabetes mellitus. Diabetologia 30:208–213
Zerbini G, Mangili R, Luzi L (1999) Higher post-absorptive C-peptide levels in Type 1 diabetic patients without renal complications. Diabet Med 16:1048
Panero F, Novelli G, Zucco C et al (2009) Fasting plasma C-peptide and micro- and macrovascular complications in a large clinic-based cohort of type 1 diabetic patients. Diabetes Care 32:301–305
Young LH, Ikeda Y, Scalia R, Lefer AM (2000) C-peptide exerts cardioprotective effects in myocardial ischemia-reperfusion. Am J Physiol Heart Circ Physiol 279:H1453–H1459
Scalia R, Coyle KM, Levine BJ, Booth G, Lefer AM (2000) C-peptide inhibits leukocyte-endothelium interaction in the microcirculation during acute endothelial dysfunction. FASEB J 14:2357–2364
Luppi P, Cifarelli V, Tse H, Piganelli J, Trucco M (2008) Human C-peptide antagonises high glucose-induced endothelial dysfunction through the nuclear factor-kappaB pathway. Diabetologia 51:1534–1543
Vish MG, Mangeshkar P, Piraino G, Denenberg A, Hake PW, O’Connor M, Zingarelli B (2007) Proinsulin c-peptide exerts beneficial effects in endotoxic shock in mice. Crit Care Med 35:1348–1355
Sima AA, Zhang W, Kreipke CW, Rafols JA, Hoffman WH (2009) Inflammation in diabetic encephalopathy is prevented by C-peptide. Rev Diabet Stud 6:37–42
Mustapha NM, Tarr JM, Kohner EM, Chibber R (2010) NADPH oxidase mitochondria-derived ROS in glucose-induced apoptosis of pericytes in early diabetic retinopathy. J Ophthalmol 2010:746978
Zhao X, Carnevale KA, Cathcart MK (2003) Human monocytes use Rac1, not Rac2, in the NADPH oxidase complex. J Biol Chem 278:40788–40792
Al-Rasheed NM, Willars GB, Brunskill NJ (2006) C-peptide signals via Galpha I to protect against TNF-alpha-mediated apoptosis of opossum kidney proximal tubular cells. J Am Soc Nephrol 17:986–995
Rimm EB, Stampfer MJ, Ascherio A, Giovannucci E, Colditz GA, Willett WC (1993) Vitamin E consumption and the risk of coronary heart disease in men. N Engl J Med 328:1450–1456
Stampfer MJ, Hennekens CH, Manson JE, Colditz GA, Rosner B, Willett WC (1993) Vitamin E consumption and the risk of coronary disease in women. N Engl J Med 328:1444–1449
Lee TC, Barshes NR, Agee EE, O’Mahoney CA, Brunicardi FC, Goss JA (2006) The effect of whole organ pancreas transplantation and PIT on diabetic complications. Curr Diab Rep 6:323–327
Shapiro AM, Ricordi C, Hering BJ et al (2006) International trial of the Edmonton protocol for islet transplantation. N Engl J Med 355:1318–1330
Mughal RS, Scragg JL, Lister P et al (2010) Cellular mechanisms by which proinsulin C-peptide prevents insulin-induced neointima formation in human saphenous vein. Diabetologia 53:1761–1771
Bugliani M, Torri S, Lupi R et al (2007) Effects of C-peptide on isolated human pancreatic islet cells. Diabetes Metab Res Rev 23:215–219
Zamzani N, Brenner C, Marzo I, Susin SA, Kroemer G (1998) Subcellular and submitochondrial mode of action of Bcl-2-like oncoproteins. Oncogene 16:2265–2282
Li ZG, Zhang W, Sima AA (2003) C-peptide enhances insulin-mediated cell growth and protection against high glucose-induced apoptosis in SH-SY5Y cells. Diabetes Metab Res Rev 19:375–385
Li ZG, Zhang W, Grunberger G, Sima A (2002) Hippocampal neuronal apoptosis in type 1 diabetes. Brain Res 946:221–231
Sheu ML, Ho FM, Yang RS et al (2005) High glucose induces human endothelial cell apoptosis through a phosphoinositide 3-kinase-regulated cyclooxygenase-2 pathway. Arterioscler Thromb Vasc Biol 25:539–545
Sima AA, Li ZG (2005) The effect of C-peptide on cognitive dysfunction and hippocampal apoptosis in type 1 diabetic rats. Diabetes 54:1497–1505
Sima AA, Zhang W, Muzik O, Kreipke CW, Rafols JA, Hoffman WH (2009) Sequential abnormalities in type 1 diabetic encephalopathy and the effects of C-peptide. Rev Diabet Stud 6:211–222
Stevens MJ, Zhang W, Li F, Sima AAF (2004) C-peptide corrects endoneurial blood flow but not oxidative stress in type 1 BB/Wor rats. Am J Physiol Endocrinol Metab 287:E497–E505
Manzella D, Ragno E, Abbatecola AM, Grella R, Paolisso G (2003) Residual C-peptide secretion and endothelial function in patients with Type II diabetes. Clin Sci (Lond) 105:113–118
Kinsella BT, Erdman RA, Maltese WA (1991) Carboxyl-terminal isoprenylation of ras-related GTP-binding proteins encoded by rac1, rac2, and ralA. J Biol Chem 266:9786–9794
Acknowledgements
This study was supported by: the Research Advisory Committee (RAC) grant from the Childrens Hospital of Pittsburgh (V. Cifarelli), the Henry Hillman Endowment Chair in Pediatric Immunology (M. Trucco) and by grants DK 024021–24 from the National Institute of Health and NIH 5 K12 DK063704 (P. Luppi and M. Trucco), and W81XWH-10-1-1055 from the Department of Defense.
Contribution statement
V.C. designed and performed the majority of experiments, analysed and interpreted the data and critically revised the article. X.G. designed and performed the TUNEL assay, analysed the images, and critically revised the article. A.S. and R.L. performed the flow cytometry and analysed the experiments for ROS detection. They also critically revised the article. M.T. conceptualised the study, interpreted the data and critically revised the article. P.L. conceptualised the study, analysed and interpreted the data and wrote the article. All authors approved the final version of the paper.
Duality of interest statement
The authors declare that there is no duality of interest associated with this manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary materials
Below is the link to the electronic supplementary material.
ESM 1
(PDF 72 kb)
Rights and permissions
About this article
Cite this article
Cifarelli, V., Geng, X., Styche, A. et al. C-peptide reduces high-glucose-induced apoptosis of endothelial cells and decreases NAD(P)H-oxidase reactive oxygen species generation in human aortic endothelial cells. Diabetologia 54, 2702–2712 (2011). https://doi.org/10.1007/s00125-011-2251-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-011-2251-0