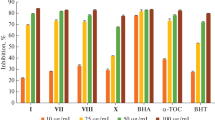
Abstract
The present study describes the synthesis and characterization of a series of novel Schiff bases derived from 2,4-dihydroxybenzaldehyde. The biological activities of the newly synthesized compounds were examined by investigating their antioxidant, antibacterial, antifungal, enzyme inhibition and DNA interaction potential. The potential of these compounds as an antioxidant was determined by 2,2-diphenylpicrylhydrazyl radical scavenging method. The antibacterial and antifungal activities of these compounds were assayed by the disk diffusion method, while the enzyme inhibition studies were carried out against acetylcholinesterase and butyrylcholinesterase. The aforementioned studies revealed that the newly synthesized Schiff bases can be used as potential inhibitors for cholinesterase. In addition, the molecular docking studies also agreed well with the experimental results with better interaction patterns in the cases of acetylcholinesterase and butyrylcholinesterase. The DNA binding interactions in these synthesized compounds was studied by the UV–Vis absorption titration method and the results of calculated thermodynamic parameters such as binding constant (K) and free energy change (ΔG) were calculated accordingly. Most of these Schiff bases displayed relatively higher positive values for K and larger negative values for ΔG, indicating efficient binding of these Schiff bases with the DNA. During the course of this study, we also carried out the computational analysis for the determination of the mode of binding of these compounds with the DNA structure.










Similar content being viewed by others
References
ACD/ChemSketch (2015) Advanced Chemistry Development, Inc., Toronto
Akhtar MS, Ismail A, Murtaza S, Tahir MN, Shamim S, Rana UA (2016) Biological and docking studies of sulfonamide derivatives of 4-Aminophenazone. J Chem Soc Pak 38(2):242–257
Bendary E, Francis RR, Ali HMG, Sarwat MI, El-Hady S (2013) Antioxidant and structure–activity relationships (SARs) of some phenolic and anilines compounds. Ann Agric Sci 58(2):173–181
Benesi H, Hildebrand J (1949) A spectrophotometric investigation of the interaction of iodine with aromatic hydrocarbons. J Am Chem Soc 71:2703–2707
Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE (2000) The protein data bank. Nucleic Acids Res 28(1):235–242
Boghaei D, Askarizadeh E, Bezaatpour A (2008) Molecular and biomolecular spectroscopy. Spectrochim Acta Part A 69:624–628
Castelli MV, Estefanía B, María Candida M, Laura AS, Francisca V, Susana AZ (2014). Novel antifungal agents: a patent review (2011-present), Expert Opin Ther Pat 24(3)
Dunbrack RL (2002) Rotamer libraries in the 21st century. Curr Opin Struct Biol 12(4):431–440
Ellman GL, Courtney KD, Andres V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Encio I, Migliaccio M, Martinez V (2004) Synthesis and cytotoxic activity of lipophilic sulphonamide derivatives of the benzo[b]thiophene 1,1-dioxide. Bioorg Med Chem 12:963–968
Fernandez-Panchon MS, Villano D, Troncoso AM, Garcia-Parrilla MC (2008) Antioxidant activity of phenolic compounds: From in vitro results to in vivo evidence. Crit Rev Food Sci Nutr 48:649–671
Florian N, Eugenie C, Cyril R, Marie T, Yvain N, Ludovic J, Pierre-Yves R (2013) Crystal structures of human cholinesterases in complex with huprine W and tacrine: elements of specificity for anti-Alzheimer’s drugs targeting acetyl- and butyryl-cholinesterase. Biochem J 453(3):393–399
Guha R, Howard MT, Hutchison GR, Rust PM, Rzepa H, Steinbeck C, Wegner J, Willighagen EL (2006) The Blue Obelisk-interoperability in chemical informatics. J Chem Inf Model 46:991–998
Gull Y, Rasool N, Noreen M, Altaf AA, Musharraf SG, Zubair M, Nasim FH, Yaqoob A, DeFeo V, Zia-Ul-Haq M (2016) Synthesis of N-(6-Arylbenzo [d] thiazole-2-acetamide derivatives and their biological activities: an experimental and computational approach. Molecules 21(3):266
Hameed A, Zehra ST, Shah SJ, Khan KM, Alharthy RD, Furtmann N, Bajorath J, Tahir MN, Iqbal J (2015) Syntheses, cholinesterases inhibition, and molecular docking studies of Pyrido [2, 3‐b] pyrazine derivatives. Chem Biol Drug Des 86(5):1115–1120
Hassan MM, Oyewale AO, Amupitan JO, Abduallahi MS, Okonkwo EM (2004) Preliminary phytochemical and antibacterial investigation of crude extracts of the root bark of Detarium microcarpum. J Chem Soc Nigeria 29:26–29
Joshi S, Vagdevi H, Vaidya V, Gadaginamath G (2008) Synthesis of new 4-pyrrol-l-yl benzoic acid hydrazide analogs and some derived oxadiazole, triazole and pyrrole ring systems: a novel class of potential antibacterial and antitubercular agents. Eur J Med Chem 43:1989–1996
Kalaivani S, Priya N, Arunachalam S (2012) Schiff Bases: facile synthesis, spectral characterization and biocidal studies. Int J Appl Bio Pharm Technol 3:219–223
Kathiravan MK, Amol BS, Aparna SC, Prashik BD, Rahul PW, Maheshwar SM, Sandeep G (2012) The biology and chemistry of antifungal agents: a review. Bioorg Med Chem 20:5678–5698
Kessler M, Ubeaud G, Jung L (2003) Anti- and pro-oxidant activity of rutin and quercetin derivatives. J Pharm Pharmacol 55:131–142
Kumar K, Keshavayya J, Rajesh T, Peethambar K (2013) Synthesis, characterization and biological activity of heterocyclic azo dyes derived from 2-aminobenzothiozole. Int J Pharm Sci 5(1):296–301
Li Y, Yang Z, Zhang H, Cao B (2003) Artemisinin derivatives bearing Mannich base group: synthesis and antimalarial activity. Bio Org Med Chem 11:4363–4368
Lipinski CA, Lombardo F, Dominy BW, Feeney PJ (1997) Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev 23:3–25
Mathew B, Vakketh SS, Kumar SS (2010) Synthesis, molecular properties and anthelmintic activity of some Schiff bases of 1, 3, 4 thiadiazole derivatives. Der Pharma Chem 2(5):337–343
Mathur A, Singh R, Yousuf S, Bhardwaj A, Verma SK, Babu P, Gupta V, Prasad G, Dua VK (2011) Antifungal activity of some plant extracts against clinical pathogens. Adv Appl Sci Res 2(2):260–264
Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ (2009) AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem 30(16):2785–2791
Mungole AJ, Awati R, Chaturvedi A, Zanwar (2010) Preliminary phytochemical screening of ipomoea obscura (L)—a hepatoprotective medicinal plant. Int J PharmTech Res 2(4):2307–2312
Murtaza S, Akhtar MS , Kanwal F, Abbas A, Ashiq S, Shamim S (2014) Synthesis and biological evaluation of Schiff bases of 4-aminophenazone as an anti-inflammatory, analgesic and antipyretic agent. J Saudi Chem Soc. doi:10.1016/j.jscs.2014.04.003.
Murtaza S, Shamim S, Kousar N, Tahir MN, Sirajuddin M, Rana UA (2016) Synthesis, biological investigation, calf thymus DNA binding and docking studies of the sulfonyl hydrazides and their derivatives. J Mol Struct 1107:99–108
Pandey S, Lakshmi V, Pandey A (2003) Biological activity of Mannich bases. Indian Pharm Sci 65:213–222
Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE (2004) UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem 25(13):1605–1612
Pizzolitto RP, Carla LB, José SD, Jimena MH, María PZ, Carina EM, Héctor RR, Julio AZ, Ana MD (2015) Inhibitory effect of natural phenolic compounds on Aspergillus parasiticus growth. J Chem 2015:7. Article ID 547925
Prior RL, Wu H, Gu L (2006) Flavonoid metabolism and challenges to understanding mechanisms of health effects. J Sci Food Agric 86:2487–2491
Raza R, Saeed A, Arif M, Mahmood S, Muddassar M, Raza A, Iqbal J (2012) Synthesis and biological evaluation of 3-thiazolocoumarinyl schiff-base derivatives as cholinesterase inhibitors. Chem Biol Drug Des 80:605–615
Shirwaikar A, Malini S, Kumari SC (2003) Protective effect of Pongamia pinnata flowers against cisplatin and gentamicin induced Dammarane triterpene saponin from Bacopa monniera nephrotoxicity in rats. Indian J Exp Biol 1:58–62
Venugopal K, Jayashree B (2008) Microwave-induced synthesis of Schiff bases of aminothiazolylbromocoumarins as antibacterials. Indian J Pharm Sci 70:88–91
Wadher S, Puranik M, Karande N, Yeole P (2009) Synthesis and biological evaluation of Schiff base of dapsone and their derivative as antimicrobial agents. Int J PharmTech Res 1:22–33
Wang J, Wang W, Kollman PA, Case DA (2006) Automatic atom type and bond type perception in molecular mechanical calculations. J Mol Graph Model 25(2):247–260
Williams WB, Cuvelier ME, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. Lebensm Wiss Technol 28(1):25–30
Wu H, Haig T, Prately J, Lemerie D, An M (2000) Allelochemicals in wheat (Triticum aestivum L.): variation of phenolic acids in root tissue. J Agri Food Chem 78(48):5321–5325
Acknowledgment
The authors gratefully acknowledged Dr. Safeer Ahmed, Quaid-i-Azam University Islamabad, Pakistan, for his help in the NMR analysis and Dr. Sajid Mahmood, University of Gujrat (UOG), Gujrat, Pakistan for biological studies. The authors extend their sincere appreciations to the Deanship of Scientific Research at King Saud University for its funding this Prolific Research Group (PRG-1437-34).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Rights and permissions
About this article
Cite this article
Murtaza, S., Abbas, A., Iftikhar, K. et al. Synthesis, biological activities and docking studies of novel 2,4-dihydroxybenzaldehyde based Schiff base. Med Chem Res 25, 2860–2871 (2016). https://doi.org/10.1007/s00044-016-1711-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-016-1711-y