Abstract
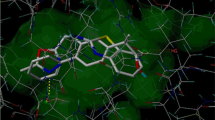
A series of new 2-substituted-N-(1,3-thiazole-2-yl)acetamide 3–7 and N-(benzo[d]thiazol-2-yl)-2-(substituted)acetamide 10–13 derivatives have been synthesized and evaluated in vivo (rat paw edema) for their anti-inflammatory activities and in silico(docking studies) to recognize the hypothetical binding motif of the title compounds with the cyclooxygenase isoenzyme (COX-2) employing GLIDE software (Schrodinger Inc.). The compounds, 10–13 were found to have good anti-inflammatory activities [around 84–93 % of the standard: indomethacin]. The binding mode of the title compounds has been proposed based on the docking studies. Further, the predicted ADME properties of all the tested compounds were found to be in the ranges as predicted by QikProp for 95 % of known oral drugs and also satisfy the Lipinski’s rule of five.




Similar content being viewed by others
References
Afshin Z, Leila N, Bahram D, Orkideh GD, Mehdi H (2007) Synthesis of 2,3-diaryl-1,3-thiazolidine-4-one derivatives as selective cyclooxygenase (COX-2) inhibitors. Bioorg Med Chem Lett 17:5634–5637
Alka B, Ruchika O, Pran Kishore D (2012) Synthesis, evaluation and docking studies on 3-alkoxy-4-methanesulfonamido acetophenone derivatives as non ulcerogenic anti-inflammatory agents. Eur J Med Chem 49:397–405
Allison MC, Howatson AG, Torrance CJ, Lee FD, Russell RI (1992) Gastrointestinal damage associated with the use of non-steroidal anti-inflammatory drugs. N Eng J Med 327:749–754
Balakumar C, Lamba P, Pran Kishore D, Lakshminarayana B, Venkat Rao K, Rajwinder K, Raghuram Rao A, Shireesha B, Narsaiah B (2010) Synthesis, anti-inflammatory evaluation and docking studies of some new fluorinated fused quinazolines. Eur J Med Chem 45:4904–4913
Balladka KS, Bettadapura GK, Chenna GD, Basavapattana RB, Hanumanthappa M (2010) Synthesis, characterization, in vitro and molecular docking studies of new 2,5-dichloro thienyl substituted thiazole derivatives for antimicrobial properties. Eur J Med Chem 45:3490–3496
Bharti SK, Nath G, Tilak R, Singh SK (2010) Synthesis, anti-bacterial and anti-fungal activities of some novel Schiff bases containing 2,4-disubstituted thiazole ring. Eur J Med Chem 45:651–660
Charles EE, Raymond ND (1995) Eicosanoids and the gastrointestinal tract. Gastroenterology 109:285–301
Franklin PX, Ajay DP, Parendu DR, Swapnil Y, Manish N, Harish P, Kamala KV, Sudarsanam V (2008) 2-Amino-5-thiazolyl motif: a novel scaffold for designing anti-inflammatory agents of diverse structures. Eur J Med Chem 43:129–134
George AK, Richard AF (2001) Evaluation and reparametrization of the OPLS-AA force field for proteins via comparison with accurate quantum chemical calculations on peptides. J Phys Chem B 105:6474–6487
GraphPad Software Inc (2007) CA, USA
Guangfu Y, Li X, Aihong Lu (2001) Synthesis and bioactivity of novel triazolo[1,5-a]pyrimidine derivatives[3]. Heteroatom Chem 12:491–496
Harvey RH (1996) Prostaglandin synthase 2. Biochem Biophys Acta 1299:125–140
Jean MD, Claudiu TS, Domenico P (2005) Adverse cardiovascular effects of the coxibs. J Med Chem 48:2251–2257
Jeffery SC, Mathew JG, Karen S, Carol MK, Yan Z, John JT (1999) Design and synthesis of sulfonyl-substituted 4,5-diarylthiazoles as selective cyclooxygenase-2 inhibitors. Bioorg Med Chem Lett 9:1167–1170
Kawamori T, Rao CV, Seibert K, Reddy BS (1998) Chemopreventive activity of celecoxib, a specific cyclooxygenase-2 inhibitor, against colon carcinogenesis. Cancer Res 58:409–412
Lisa EY, Dan AD (2010) Posttranscriptional regulation of cyclooxygenase 2 expression in colorectal cancer. Curr Colorectal Cancer Rep 6:60–67
Maestro version 9.4, and Glide v5.9, (2013–1) Schrödinger, LLC, New York, NY
Mamta SD, Pran Kishore D, Renu C, Tejvir S, Maninder K (2013) Synthesis, evaluation and molecular docking studies of cycloalkyl/aryl-3,4,5-trimethylgallates as potent non-ulcerogenic and gastroprotective anti-inflammatory agents. Med Chem Res doi:10.1007/s00044-013-0620-6
Masakazu B, Hiroaki T, Takeo K, Mitsuru T, Kiyotaka S, Akihiko W, Takanari T (1998) Novel antiallergic and antiinflammatory agents. Part I: synthesis and pharmacology of glycolic amide derivatives. Bioorg Med Chem 6:1069–1076
Michel T, Christine B, Chi CC, Wanda AC, Jacques YG, Robert G, Gillian G, Stacia K, Cheuk KL, Yves L, Chun SL, Gary PO, Denis R, Patrick R, Zhaoyin W, Li**g X, Petpiboon P (1997) Synthesis and biological evaluation of 5,6-diarylimidazo[2,1-b]thiazole as selective COX-2 inhibitors. Bioorg Med Chem Lett 7:47–52
Narendra S, Uma Shankar S, Niranjan S, Sushil K, Umesh KS (2010) Synthesis and antimicrobial activity of some novel 2-amino thiazole derivatives. J Chem Pharm Res 2:691–698
Paul SA, Kimberly AS, Michael G, Eric P, Mary S, Kenneth ID, Martin RF, Shelia J, Ronald GT, Leon JT (2003) Effects of rofecoxib or naproxen vs placebo on alzheimer disease progression a randomized controlled trial. JAMA 289:2819–2826
Pawan KS, Sawhney SN (1997) Potent antiinflammatory 3-thiazole-4(5)-acetic acids of 1,2-benzisothiazole. Bioorg Med Chem Lett 7:2427–2430
Prakash K, Mari SK, Dasappa JP, Manjathuru M, Bantwal SH, Nalilu SK (2008) Synthesis of some novel 2,4-disubstituted thiazoles as possible antimicrobial agents. Eur J Med Chem 43:261–267
QikProp, version 3.6, (2013) Schrödinger, LLC, New York
Richard AF, Jay LB, Robert BM, Thomas AH, Jasna JK, Daniel TM, Matthew PR, Eric HK, Mee S, Jason KP, David ES, Perry F, Peter SS (2004) Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J Med Chem 47:1739–1749
Rosaria O, Rosanna M, Maria LB, Giuseppe B, Archimede R, Antonietta R, Giuseppa C, Rosanna DP, Lidia S, Salvatore C, Maria GV (2005) 5-Arylidene-2-imino-4-thiazolidinones: design and synthesis of novel anti-inflammatory agents. Bioorg Med Chem 13:4243–4252
Sara A, Afshin Z (2010) Design, synthesis and biological evaluation of new (E)-and (Z)-1,2,3-triaryl-2-propen-1-ones as selective COX-2 inhibitors. Eur J Med Chem 45:4013–4017
Shigeru K, Tetsunari T, Ken-ichi Y (2007) Cyclooxygenase-2 and tumor biology. Adv Clin Chem 43:59–78
Stefano F, Rosaria M, Mariarosaria B, Giuseppe C (2001) Dual inhibitors of cyclooxygenase and 5-lipoxygenase. A new avenue in anti-inflammatory therapy? Biochem Pharmacol 62:1433–1438
Taketo MM (1998) COX-2 and colon cancer. Inflamm Res 47:112–116
Van Gool WA, Aisen PS, Eikelenboom P (2003) Anti-inflammatory therapy in Alzheimer’s disease: is hope still alive? J Neurol 250:788–792
Vane JR, Bakhle YS, Botting RM (1998) Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol 38:97–120
William KH (2008) The many roles for fluorine in medicinal chemistry. J Med Chem 51:4359–4369
William LS, David LD (1996) Prostaglandin endoperoxide H synthases-1 and -2. Adv Immunol 62:167–215
Winter CA, Risely EA, Nuss GW (1962) Carrageenin-induced edema in hind paw of the rat as an assay for antiiflammatory drugs. Proc Soc Exp Biol Med 111:544–547
**e WL, Chipman JG, Robertson DL, Erikson RL, Simmons DL (1991) Expression of a mitogen-responsive gene encoding prostaglandin synthase is regulated by mRNA splicing. Proc Natl Acad Sci USA 88:2692–2696
Acknowledgments
The author PKD is thankful to CSIR, New Delhi for awarding the Senior Research Fellowship [Sanction No. 09/135/(0534)/2008/EMR-I (18/03/2008)]. The authors thankfully acknowledge the Chairman, UIPS, Chandigarh for providing the facilities. Authors are also thankful to Dr. Kanwaljit Chopra, Pharmacology Division and Dr. Maninder Karan Vasisht, Pharmacognosy Division, UIPS, PU for valuable help during pharmacological studies. Authors profusely thank to Avtar Singh, SAIF (CIL), PU for carrying out the NMR studies. Author PKD is thankful to Pharmaceutical Chemistry Division, School of Pharmacy, IMU, Malaysia for providing the facilities to carry out the docking studies. Authors are also thankful to Mr. Raghurangaswamy, Executive Director, Schrodinger, India for providing the evaluation license (14 Jun–16 July, 2013) to carry out the molecular modeling studies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Deb, P.K., Kaur, R., Chandrasekaran, B. et al. Synthesis, anti-inflammatory evaluation, and docking studies of some new thiazole derivatives. Med Chem Res 23, 2780–2792 (2014). https://doi.org/10.1007/s00044-013-0861-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-013-0861-4