Abstract
Objectives and design
Transplanted cell survival might greatly improve the therapeutic efficacy of cell therapy. Diazoxide (DZ), a highly selective mitochondrial ATP-sensitive potassium channel opener, is known to suppress cell apoptosis and protect cells in oxidative stressed ischemic environment. We explored the mechanisms involved in DZ pre-treatment-induced anti-apoptotic effect on L6 skeletal myoblast (SKM).
Materials and methods
L6 SKMs were divided into control group, H2O2 group, DZ + H2O2 group and DZ + LY + H2O2 group. Treatments of 400 μmol/L H2O2 for 24 h alone, or after 200 μmol/L DZ pre-treatment for 30 min, or after DZ and 50 μmol/L LY294002 co-administration for 30 min were performed. The cell apoptosis rates were assessed by flow cytometric analysis. The changes of mitochondrial membrane potential were determined by JC-1 mitochondrial staining. The activation of phosphatidylinositol-3 kinase (PI3K)/Akt, caspase-9 and caspase-3 was detected by western blot.
Results
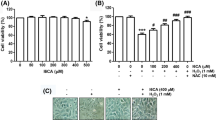
Compared with the H2O2 group, DZ pre-treatment protected cells from H2O2-induced damage, increased Akt phosphorylation, prevented mitochondrial membrane depolarization as well as the activation of caspase-9 and caspase-3 and decreased the cell apoptosis rate. However, the DZ-induced cytoprotective and anti-apoptosis effects were partly inhibited by co-administration of a PI3K inhibitor, LY294002.
Conclusions
These data suggest that DZ pre-treatment contributes to protection of L6 SKMs against apoptosis at least partly by activating the PI3K/Akt pathway and subsequently inhibiting the mitochondrial-mediated caspase-dependent apoptotic signalling pathway.





Similar content being viewed by others
Abbreviations
- SKM:
-
Skeletal myoblast
- DZ:
-
Diazoxide
- PI3K:
-
Phosphatidylinositol-3 kinase
- mitoKATP channel:
-
Mitochondrial ATP-sensitive potassium channels
- ROS:
-
Reactive oxygen species
- MTT:
-
Methyl thiazolyl tetrazolium
- Annexin V-FITC/PI:
-
Annexin V-fluorescein isothiocyanate/propidium iodide
- IOD:
-
Integral optical density
- JC-1:
-
5,5′,6,6′-tetrachloro1,1′,3,3′-tetraethyl-benzimidazolylcarbocyanine iodide
References
Yu SP, Wei Z, Wei L. Preconditioning strategy in stem cell transplantation therapy. Transl Stroke Res. 2013;4(1):76–88. doi:10.1007/s12975-012-0251-0.
Hua P, Tao J, Liu JY, Yang SR. Cell transplantation into ischemic myocardium using mesenchymal stem cells transfected by vascular endothelial growth factor. Int J Clin Exp Pathol. 2014;7(11):7782–8.
Niagara MI, Haider H, Jiang S, Ashraf M. Pharmacologically preconditioned skeletal myoblasts are resistant to oxidative stress and promote angiomyogenesis via release of paracrine factors in the infarcted heart. Circ Res. 2007;100(4):545–55. doi:10.1161/01.RES.0000258460.41160.ef.
Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105(1):93–8.
Pagani FD, DerSimonian H, Zawadzka A, Wetzel K, Edge AS, Jacoby DB, et al. Autologous skeletal myoblasts transplanted to ischemia-damaged myocardium in humans. Histological analysis of cell survival and differentiation. J Am Coll Cardiol. 2003;41(5):879–88.
Haider H, Ashraf M. Strategies to promote donor cell survival: combining preconditioning approach with stem cell transplantation. J Mol Cell Cardiol. 2008;45(4):554–66. doi:10.1016/j.yjmcc.2008.05.004.
Bousselmi R, Lebbi MA, Ferjani M. Myocardial ischemic conditioning: physiological aspects and clinical applications in cardiac surgery. J Saudi Heart Assoc. 2014;26(2):93–100. doi:10.1016/j.jsha.2013.11.001.
Maslov LN, Barzakh EI, Krylatov AV, Chernysheva GA, Krieg T, Solenkova NV, et al. Opioid peptide deltorphin II simulates the cardioprotective effect of ischemic preconditioning: role of delta(2)-opioid receptors, protein kinase C, and K(ATP) channels. Bull Exp Biol Med. 2010;149(5):591–3.
Li H, Yang T, Long Z, Cheng J. Effect of mitochondrial ATP-sensitive potassium channel opening on the translocation of protein kinase C epsilon in adult rat ventricular myocytes. Genet Mol Res. 2014;13(2):4516–22. doi:10.4238/2014.June.17.3.
Otani H. Ischemic preconditioning: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2008;10(2):207–47. doi:10.1089/ars.2007.1679.
Suzuki K, Smolenski RT, Jayakumar J, Murtuza B, Brand NJ, Yacoub MH. Heat shock treatment enhances graft cell survival in skeletal myoblast transplantation to the heart. Circulation. 2000;102(19 Suppl 3):III216–21.
Shao SX, Zhang L, Chen HX, Liu Y, Zhang JP, Chen W, et al. Diazoxide pretreatment enhances L6 skeletal myoblast survival and inhibits apoptosis induced by hydrogen peroxide. Anat Rec (Hoboken). 2012;295(4):632–40. doi:10.1002/ar.22410.
Salvioli S, Ardizzoni A, Franceschi C, Cossarizza A. JC-1, but not DiOC6(3) or rhodamine 123, is a reliable fluorescent probe to assess delta psi changes in intact cells: implications for studies on mitochondrial functionality during apoptosis. FEBS Lett. 1997;411(1):77–82.
Pouzet B, Vilquin JT, Hagege AA, Scorsin M, Messas E, Fiszman M, et al. Factors affecting functional outcome after autologous skeletal myoblast transplantation. Ann Thorac Surg. 2001;71(3):844–50.
Rocha M, Apostolova N, Hernandez-Mijares A, Herance R, Victor VM. Oxidative stress and endothelial dysfunction in cardiovascular disease: mitochondria-targeted therapeutics. Curr Med Chem. 2010;17(32):3827–41.
Djordjevic VB, Zvezdanovic L, Cosic V. Oxidative stress in human diseases. Srp Arh Celok Lek. 2008;136(Suppl 2):158–65.
Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87(1):99–163. doi:10.1152/physrev.00013.2006.
Simerabet M, Robin E, Aristi I, Adamczyk S, Tavernier B, Vallet B, et al. Preconditioning by an in situ administration of hydrogen peroxide: involvement of reactive oxygen species and mitochondrial ATP-dependent potassium channel in a cerebral ischemia-reperfusion model. Brain Res. 2008;1240:177–84. doi:10.1016/j.brainres.2008.08.070.
Choi EM. Protective effect of diazoxide against antimycin A-induced mitochondrial dysfunction in osteoblastic MC3T3-E1 cells. Toxicol In Vitro. 2011;25(8):1603–8. doi:10.1016/j.tiv.2011.06.004.
Robin E, Simerabet M, Hassoun SM, Adamczyk S, Tavernier B, Vallet B, et al. Postconditioning in focal cerebral ischemia: role of the mitochondrial ATP-dependent potassium channel. Brain Res. 2011;1375:137–46. doi:10.1016/j.brainres.2010.12.054.
Shin BS, Kim HG, Choi OH. Mitochondrial channel opener diazoxide attenuates hypoxia-induced sFlt-1 release in human choriocarcinoma cells. J Menopausal Med. 2014;20(1):21–31. doi:10.6118/jmm.2014.20.1.21.
Kersten JR, Gross GJ, Pagel PS, Warltier DC. Activation of adenosine triphosphate-regulated potassium channels: mediation of cellular and organ protection. Anesthesiology. 1998;88(2):495–513.
Szewczyk A, Marban E. Mitochondria: a new target for K channel openers? Trends Pharmacol Sci. 1999;20(4):157–61.
Cui X, Wang H, Guo H, Wang C, Ao H, Liu X, et al. Transplantation of mesenchymal stem cells preconditioned with diazoxide, a mitochondrial ATP-sensitive potassium channel opener, promotes repair of myocardial infarction in rats. Tohoku J Exp Med. 2010;220(2):139–47.
Dhanasekaran A, Gruenloh SK, Buonaccorsi JN, Zhang R, Gross GJ, Falck JR, et al. Multiple antiapoptotic targets of the PI3K/Akt survival pathway are activated by epoxyeicosatrienoic acids to protect cardiomyocytes from hypoxia/anoxia. Am J Physiol Heart Circ Physiol. 2008;294(2):H724–35. doi:10.1152/ajpheart.00979.2007.
Abe E, Fujiki M, Nagai Y, Shiqi K, Kubo T, Ishii K, et al. The phosphatidylinositol-3 kinase/Akt pathway mediates geranylgeranylacetone-induced neuroprotection against cerebral infarction in rats. Brain Res. 2010;1330:151–7. doi:10.1016/j.brainres.2010.02.074.
Xue Y, **e N, Lin Y, Xu J, Han Y, Wang S, et al. Role of PI3K/Akt in diazoxide preconditioning against rat hippocampal neuronal death in pilocarpine-induced seizures. Brain Res. 2011;1383:135–40. doi:10.1016/j.brainres.2011.01.037.
Ahmad N, Wang Y, Haider KH, Wang B, Pasha Z, Uzun O, et al. Cardiac protection by mitoKATP channels is dependent on Akt translocation from cytosol to mitochondria during late preconditioning. Am J Physiol Heart Circ Physiol. 2006;290(6):H2402–8. doi:10.1152/ajpheart.00737.2005.
Su C, **a T, Ren S, Qing S, **g D, Lian H, et al. Effect of diazoxide preconditioning on cultured rat myocardium microvascular endothelial cells against apoptosis and relation of PI3K/Akt pathway. Balk Med J. 2014;31(1):83–7. doi:10.5152/balkanmedj.2013.8458.
Afzal MR, Haider H, Idris NM, Jiang S, Ahmed RP, Ashraf M. Preconditioning promotes survival and angiomyogenic potential of mesenchymal stem cells in the infarcted heart via NF-kappaB signaling. Antioxid Redox Signal. 2010;12(6):693–702. doi:10.1089/ars.2009.2755.
Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307(5712):1098–101. doi:10.1126/science.1106148.
Siragusa M, Katare R, Meloni M, Damilano F, Hirsch E, Emanueli C, et al. Involvement of phosphoinositide 3-kinase gamma in angiogenesis and healing of experimental myocardial infarction in mice. Circ Res. 2010;106(4):757–68. doi:10.1161/CIRCRESAHA.109.207449.
Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91(2):231–41.
Matsuzaki H, Tamatani M, Mitsuda N, Namikawa K, Kiyama H, Miyake S, et al. Activation of Akt kinase inhibits apoptosis and changes in Bcl-2 and Bax expression induced by nitric oxide in primary hippocampal neurons. J Neurochem. 1999;73(5):2037–46.
Kennedy SG, Kandel ES, Cross TK, Hay N. Akt/Protein kinase B inhibits cell death by preventing the release of cytochrome c from mitochondria. Mol Cell Biol. 1999;19(8):5800–10.
Fujita E, **bo A, Matuzaki H, Konishi H, Kikkawa U, Momoi T. Akt phosphorylation site found in human caspase-9 is absent in mouse caspase-9. Biochem Biophys Res Commun. 1999;264(2):550–5. doi:10.1006/bbrc.1999.1387.
Acknowledgments
This research was supported by the Nature Science Foundation of Hebei Province, No. H2015206440.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Yoshiya Tanaka.
Rights and permissions
About this article
Cite this article
Chen, W., Liu, Y., Xue, G. et al. Diazoxide protects L6 skeletal myoblasts from H2O2-induced apoptosis via the phosphatidylinositol-3 kinase/Akt pathway. Inflamm. Res. 65, 53–60 (2016). https://doi.org/10.1007/s00011-015-0890-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-015-0890-1