Abstract
Background
Disease-modifying therapies (DMTs) for multiple sclerosis (MS) target immunity and have the potential to increase the risk of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection and alter its clinical course. We assessed these risks in patients with MS (PwMS).
Objective
The objective of this study was to describe the overall risk of coronavirus disease 2019 (COVID-19) infection, severe disease course, and potential population-level predictors of COVID-19 infection in PwMS, and to provide a context using a cohort of patients with systemic lupus erythematosus (SLE). In addition, the association of different MS DMTs with the incidence and clinical course of COVID-19 was evaluated. Safety data from the Biogen Global Safety Database are also presented on reported cases of COVID-19 in patients treated with Biogen MS therapies.
Methods
The IBM® Explorys electronic health record database of > 72,000,000 patients from US healthcare networks identified patients with MS or SLE, with and without polymerase chain reaction-confirmed COVID-19. COVID-19 cumulative incidence, hospitalization, and deaths among DMT classes were compared using logistic regression (adjusted for age, sex, body mass index, comorbidities, and race/ethnicity). As a secondary data source to assess safety data, COVID-19 reports for Biogen MS therapies were extracted and described from Biogen’s Global Safety Database.
Results
30,478 PwMS with an open DMT prescription were identified within Explorys; 344 were COVID-19 positive. The most significant risk factors for acquiring COVID-19 were comorbidity score ≥ 1, body mass index ≥ 30, and Black/African ancestry. Similar risk factors were also identified for patients with SLE. Patients with MS were less likely to develop COVID-19 when treated with interferons (0.61%) and glatiramer acetate (0.51%), vs all other MS DMTs (both p < 0.001); anti-CD20 therapy was associated with the highest risk (3.45%; p < 0.0001). In the Biogen Global Safety Database, we identified 1217 patients who were COVID-19 positive treated with intramuscular interferon beta-1a, peginterferon beta-1a, natalizumab, dimethyl fumarate, diroximel fumarate, or fampridine.
Conclusions
Comorbidities, obesity, and Black/African ancestry, but not age, were associated with a higher risk of SARS-CoV-2 infection in PwMS. Interferons and glatiramer acetate were associated with a reduced COVID-19 risk, whereas anti-CD20 therapies were associated with an increased risk, within the treated MS cohort. COVID-19 safety reports for patients receiving Biogen MS therapies were consistent with the Explorys database and MS literature, illustrating the replicability and power of this approach.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In this analysis using US electronic health records data, there was a similar risk of develo** coronavirus disease 2019 (COVID-19) for patients with multiple sclerosis (MS) and for patients with systemic lupus erythematosus, another chronic autoimmune disease. |
Patients treated with interferons or glatiramer acetate were less likely to develop COVID-19, and patients treated with anti-CD20 therapies were more likely to develop COVID-19, than patients with MS who were treated with all other disease-modifying therapies. |
Comorbidities, obesity, and Black/African ancestry were associated with a higher risk of infection with severe acute respiratory syndrome coronavirus-2 in patients with MS. |
In the Biogen Global Safety Database, case reports of the clinical course of COVID-19 in patients with MS treated with interferons, natalizumab, fumarates, or fampridine were consistent with the general population. |
1 Introduction
An outbreak of pneumonia in Wuhan, China, in December 2019 crossed territorial boundaries and developed into a global pandemic that threatened the well-being of healthy and, especially, vulnerable populations [1,2,33, 38].
2.2.2 Biogen GSD
Descriptive analysis of reports of COVID-19 in PwMS receiving Biogen therapies within the Biogen GSD include serious and non-serious events including fatal outcomes and cumulative reporting rate (95% CI) per 1000 patient-years.
3 Results
3.1 IBM Explorys Real-World Dataset
3.1.1 Incidence of and Risk Factors for COVID-19 in PwMS
In the IBM Explorys database, 153,663 patients had a diagnosis of MS. Of the PwMS, 30,478 had a recorded open prescription for a DMT. The incidence of COVID-19 was 0.50% (n = 761) for the overall MS population and 1.13% (n = 344) in patients receiving DMTs. COVID-19 incidence was ~ 0.4% in wMS with no open DMT prescription, a population enriched for no treatment, yet likely containing some receiving treatment because of incomplete EHR documentation or current inactivity in the EHR system. This limitation precludes any definitive comparison between patients receiving and not receiving DMTs.
In the Explorys database, PwMS were primarily female (74%), white (75%), and non-Hispanic (68%). There were 17,661 (11%) patients identified as having Black/African ancestry and 4789 (3%) identified as Hispanic; the others were of unknown race or ethnicity. Patients with MS were primarily 40–49 (20%), 50–59 (21%), and 60–69 (27%) years of age. BMI was ≥ 30 kg/m2 in 29% of PwMS. Fifty-four percent of PwMS had a comorbidity score ≥ 1. Of the individual comorbidities, 43% were diagnosed with hypertension, 18% with diabetes, 15% with cardiovascular disease, 19% with cancer, 12% with chronic obstructive pulmonary disease, and 6% with chronic kidney disease.
Patients with MS at a higher risk of contracting COVID-19 included those with a comorbidity score ≥ 1, with higher BMI (≥ 30 kg/m2), and who had Black/African ancestry (Table 1). Patients with comorbidities such as hypertension, diabetes, chronic obstructive pulmonary disease, and chronic kidney disease were at a higher risk of contracting COVID-19 (Table 1).
3.1.2 Impact of MS DMTs on the Incidence of COVID-19
Patients with MS with an open prescription of an MS DMT in the Explorys database were primarily female (75%), white (78%), and non-Hispanic (75%). In total, there were 3780 (12%) patients who identified as having Black/African ancestry and 836 (3%) who identified as Hispanic; the others were of unknown race or ethnicity. Patients with MS receiving DMT were primarily 40–49 (24%), 50–59 (25%), and 60–69 (26%) years of age. BMI was ≥ 30 kg/m2 in 36% of PwMS. Fifty-two percent of PwMS receiving DMT had a comorbidity score ≥ 1; 40% were diagnosed with hypertension, 15% with diabetes, 11% with cardiovascular disease, 14% with cancer, 8% with chronic obstructive pulmonary disease, and 5% with chronic kidney disease. The crude risk of develo** COVID-19 with different MS DMTs (categorized by proposed mechanism of action) was as follows: anti-CD20, 3.45%; anti-VLA-4, 1.35%; S1P modulators, 1.07%; fumarates, 1.01%; dihydro-orotate dehydrogenase inhibitors, 0.90%; IFNs, 0.61%; and GA, 0.51% (Table 2).
The odds of develo** COVID-19 relative to fumarates were higher for anti-CD20 therapies (OR, 3.25 [95% CI 2.31–4.64]; p < 0.0001) (Fig. 2). Anti-CD20 therapies also had a higher risk of develo** COVID-19 when compared with anti-VLA-4 antibodies (OR, 2.32 [95% CI 1.56–3.57]; p < 0.0001) and IFNs (OR, 4.65 [95% CI 3.23–6.82]; p < 0.0001). The odds of develo** COVID-19 were lower in patients treated with GA compared with fumarates (p = 0.006) and to anti-VLA-4 antibodies (p < 0.002). There was no difference in risk with GA compared to IFNs. The odds of COVID-19 infection relative to IFNs was higher in patients receiving anti-VLA-4 antibodies (OR, 2.00 [95% CI 1.21–3.27]; p = 0.005) and S1P modulators (OR, 1.65 [95% CI 1.01–2.68]; p = 0.041). However, the difference in risk was not significant when fumarates were compared to anti-VLA-4 antibodies (p = 0.159) or S1P modulators (p = 0.543).
Odds of develo** coronavirus disease 2019 among patients with multiple sclerosis by disease-modifying therapy class. Multivariate logistic regression analyses were adjusted for age, sex, race, and body mass index and for comorbidities such as hypertension, diabetes, cardiovascular disease, cancer, chronic obstructive pulmonary disease, and chronic kidney disease. The adjusted odds ratio (OR) estimates and 95% confidence intervals (CIs) based on profile penalized likelihood estimates are presented. Sample size for disease-modifying therapy use: dihydro-orotate dehydrogenase (DHO-DH) inhibitor, n = 1446; interferon (IFN)-β, n = 6509; glatiramer acetate, n = 6840; sphingosine-1-phosphate receptor (S1PR) modulator, n = 2699; anti-CD20, n = 3568; fumarate, n = 4439; anti-VLA-4, n = 2080. Disease-modifying therapies prescribed for multiple sclerosis were categorized by mechanism of action, as described in Sect. 2.2.1
Compared to the aggregate of all other DMT-treated PwMS (0.82% [221/26,910]), the risk of develo** COVID-19 was higher in anti-CD20-treated patients (3.45% [123/3568]; p < 0.0001). COVID-19 was less likely to develop in PwMS who were prescribed IFNs (0.61% [40/6509] vs 1.27% [304/23,969]; p < 0.0001) and GA (0.51% [35/6840] vs 1.31% [309/23,638]; p < 0.0001) relative to PwMS prescribed all other MS DMTs.
3.1.3 Incidence of COVID-19-Related Hospitalization and Death in PwMS
The risk of hospitalization due to COVID-19 in PwMS with an open DMT prescription was 21.5% (n = 74/344). Deaths due to COVID-19 was 4.2% (n = 32/761) in the overall MS population and 3.5% (n = 12/344) in PwMS with an open DMT prescription (Fig. 3). In PwMS with an open DMT prescription, 25.3% (n = 23/91) of PwMS of Black/African ancestry were hospitalized with COVID-19 vs 74.7% (n = 68/91) not hospitalized. In comparison, 19.6% (n = 46/235) of white PwMS were hospitalized with COVID-19 vs 80.4% (n = 189/235) not hospitalized. In PwMS with a higher BMI (≥ 30 kg/m2), 25.5% (n = 40/157) were hospitalized vs 74.5% (n = 117/157) not hospitalized. In comparison, in patients with BMI < 30 kg/m2, 18.3% (n = 34/186) were hospitalized vs 81.7% (n = 152/186) not hospitalized (Table 3). Patients with a higher comorbidity score (≥ 1) had a higher risk of hospitalization due to COVID-19, and an increasing comorbidity score was associated with an increased risk of hospitalization due to COVID-19 (Table 3). The risk of COVID-19-related hospitalization by DMT use (Table 4) was similar to risk of COVID-19 incidence (Table 2).
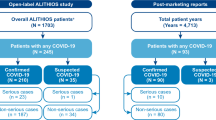
Cumulative incidence of coronavirus disease 2019 (COVID-19), and hospitalizations, and deaths due to COVID-19 in patients with multiple sclerosis (MS) or systemic lupus erythematosus (SLE). The cumulative incidence was calculated for patients with MS or SLE with an open prescription for a disease-modifying therapy based on the IBM Explorys database as of 30 November 2020. Percentage and sample size are shown
The number of deaths from COVID-19 was small in PwMS treated with DMTs (n = 12) and in the overall MS population (n = 32). However, a higher comorbidity score and older age may be associated with an increased risk (data not shown).
3.1.4 Incidence of COVID-19 in Patients with SLE
The incidence of COVID-19 cases, hospitalization, and death in patients with SLE in the IBM Explorys database as of 30 November 2020 was analyzed. In total, 166,924 patients had a diagnosis of SLE; 58,466 had an open prescription for a DMT. The incidence of develo** COVID-19 was 1.19% (n = 693) in patients with SLE with an open prescription, 0.61% (n = 1021) for the overall SLE population, and 0.3% for those with no open prescription. Patients with SLE who had comorbidities such as hypertension, diabetes, chronic kidney disease, and cancer were at greater risk of contracting COVID-19. Additionally, patients with SLE with a comorbidity score ≥ 1, higher BMI (≥ 30 kg/m2), who were older, and with Black/African ancestry had a higher risk of contracting COVID-19 (Table 1 of the Supplementary Information). Among patients with COVID-19, the risk of hospitalization due to COVID-19 was 32% (n = 225/693) in patients with SLE with an open DMT prescription (Table 2 of the Supplementary Information). Deaths due to COVID-19 were low for patients with SLE with an open DMT prescription (4.3%; n = 30/693) (Fig. 3) and for patients with SLE in the overall database population (3.8%; n = 39/1021; data not shown). However, chronic kidney disease, hypertension, diabetes, and older age may be associated with increased risk (data not shown).
3.2 Biogen GSD
The Biogen GSD contains data on five MS DMTs belonging to different drug classes, including fumarates (dimethyl fumarate, diroximel fumarate), anti-VLA-4 antibodies (natalizumab), and IFNs (intramuscular IFNβ-1a, pegIFNβ-1a), as well as a symptomatic therapy (fampridine). As of 3 December 2020, 1.59 million patients have been treated with one of these therapies. This is the most comprehensive global safety database on MS therapies in the industry and provides a unique opportunity to review the impact of COVID-19 on patients treated with DMTs.
Between 1 January and 3 December 2020, Biogen received 1217 case reports of COVID-19 in patients treated with a product in the Biogen MS portfolio (Table 3 of the Supplementary Information). Of these 1217 reports of COVID-19 globally, 578 were treated with fumarates, 464 with natalizumab, 169 with IFNs, and six with fampridine. Two hundred and seventy-one COVID-19 events were deemed as serious and 946 as non-serious as per standard International Conference on Harmonisation definitions. Data on cumulative exposure to different DMT classes and safety information are reported in the context of their use as of 31 October 2020 (Table 5). All reported cases had clinical courses consistent with the COVID-19 cases recently described in the general population. Cumulative reporting rates for COVID-19 (per 1000 patient-years; 95% CI) were 0.578 (0.532–0.627) for fumarates, 0.544 (0.495–0.593) for natalizumab, 0.059 (0.051–0.069) for IFNs, and 0.017 (0.006–0.037) for fampridine.
4 Discussion
Emerging registry and survey data indicate that the MS population may not have an increased risk of COVID-19 [17, 18, 30, 31, 39]. However, MS-associated disability and DMT therapy have been reported to be independent predictors of a higher risk of COVID-19 infection [26, 32]. Variation in COVID-19 risk may be due to differences in MS patient populations, such as progressive disease, older patients, and severe disability, where an effective DMT may be unavailable. Recent guidelines argue that continuing DMT therapy can prevent disease progression and discuss the benefit vs risk of ongoing DMT therapy in the context of local COVID-19 infections [12].
Epidemiological data reveal disparities in COVID-19 prevalence and disease outcomes among different racial and ethnic subgroups [27, 29,30,31,32]. In the USA, the rate of COVID-19 cases and COVID-19-associated mortality in patients with Black/African ancestry and Hispanic patients is disproportionately higher than their representation in the overall population [27]. Patients with MS with Black/African ancestry in this cohort, though limited in number, had a higher incidence of COVID-19 than PwMS with no Black/African ancestry [27, 28]. Similar trends were observed for hospitalization due to COVID-19. COVID-19 incidence trended higher in a similar manner for Hispanic patients. However, demographic data in the Explorys database were incomplete in many cases. Data sets and registries that focus on engagement with minority populations and that capture comprehensive race and ethnicity-based data in the context of COVID-19 incidence, outcomes, and access to healthcare and vaccination are needed.
The incidence of develo** COVID-19 in PwMS with an open DMT prescription (1.13%) is equivalent to incidence in patients with SLE with an open DMT prescription (1.19%). The varied immune effects and mechanisms of action of MS DMTs likely cause variation in the general risk of infection, consequent disease severity, and virus-induced MS reactivation/inflammation [23, 33, 40]. Real-world COVID-19 registries such as MuSC-19, COVISEP, and the MS Global Data Sharing Alliance indicate that IFNs and GA are associated with a reduced risk of develo** COVID-19 (relative to other DMTs), while anti-CD20 therapies are associated with an increased risk for COVID-19 [12, 16, 23, 33, 41, 42]. Our analysis shows similar trends in the risk of COVID-19.
The risk of infection with different DMT classes was studied in relation to fumarates, given that fumarates have been identified in prior studies as one of the most prescribed class of DMT for PwMS [33, 43]. Previous analyses showed a neutral impact of fumarates on the COVID-19 infection rate and are consistent with the data reported in this study [33, 38]. The incidence of COVID-19 in patients treated with fumarates was 1.01%, and the overall MS population with an open DMT prescription was similar at 1.13%. While fumarates may decrease lymphocyte counts [44, 45], patients treated with fumarates have functional T and B cells [46], have stable serum IgA, IgG, IgM, and IgG1–4 over 2 years of treatment, and have adequate seroprotective responses to inactivated vaccines [47]. These and other analyses suggest no impairment of function despite a decrease in absolute lymphocyte count [48]. When stratified by absolute lymphocyte count, there is no increase in infection, with the exception of PML [44]. In addition to the effect of fumarate treatment on immune cells, S1P receptor modulator treatment may reduce lymphocyte counts [49] and anti-CD20 treatment is associated with an increased risk of hypogammaglobulinemia [50]. While the effects listed above are important and relevant in the context of COVID-19, this current study was not designed to directly assess the biological effects of the MS DMTs. In our current readout, when compared to fumarates, the increased risk of COVID-19 infection with anti-CD20 therapies remained consistent and statistically significant relative to IFNs and to anti-VLA-4 antibodies.
It is expected that IFNs may affect the COVID-19 risk, given that IFNs are antiviral agents [24, 33, 51]. Recent reports of severe cases of COVID-19 among patients with mutations in type I IFN immunity [52], low expression of the IFN receptor gene IFNAR2 [53], and the presence of anti-IFN neutralizing antibodies [54] suggest that type I IFNs are likely to enhance protective immunity against SARS-CoV-2. However, available clinical trial data suggest that IFNs (used alone or in combination with antiviral agents) have mixed results in patients with SARS-CoV-2 in alleviating symptoms and shortening the duration of both viral shedding and hospital stays [55,56,57,58,59]. Although the lower risk of infection observed with GA could be associated with indirect effects on the immune system, the impact of GA-induced immune alterations is still undefined [55,56,57,58]. It remains to be determined whether the reduced risk during IFN and GA therapy is driven by modification of immunity or is from differences in patient and disease characteristics (i.e., these DMTs are prescribed for patients who are earlier in disease evolution, have milder disease activity, or less disability).
The risk of COVID-19-related hospitalization by DMT use (Table 4) was similar to the trends observed in the MuSC-19 study and MS Global Data Sharing Alliance databases [33, 38]. Given the overall low reports of hospitalization and deaths in our dataset, it is not possible to draw strong conclusions about the effect of different DMT classes on these outcomes. Multiple sclerosis-associated disability has been reported as an independent predictor of a higher risk of infection. Patients with MS who are not receiving DMT treatment may have a greater risk of infection and severe disease course of COVID-19 than those receiving therapy [26, 32]. This may potentially be associated with inherent differences in patient populations, for example, untreated PwMS may be individuals with progressive disease, older patients, or those with a severe disability, where an effective DMT option may be unavailable. Simpson-Yap et al. and Sormani et al. reported that patients receiving anti-CD20 therapies have an increased severity of COVID-19, with more hospitalizations (intensive care unit admission and/or need for ventilation) relative to other DMTs such as dimethyl fumarate and natalizumab [33, 38]. Anti-CD20 therapies impact the humoral immune response, decrease levels of IgG and IgM over time, attenuate response to inactivated vaccines, and may have additional immunoregulatory effects that modify responses to COVID-19 [59,60,61].
The SARS-CoV-2 pandemic changed patient care and DMT use in MS [62]. In some cases, treatment protocols were adjusted to postpone or discontinue the administration of some DMTs. Recent updates to US and international MS guidelines underscore the importance of continuing DMT treatment and evaluating the risk of infection with different DMTs [12, 63, 64].
The Biogen GSD represents a useful platform for analyzing reported COVID-19-related events in 1.59 million patients treated across the portfolio of six Biogen MS therapies. COVID-19 cases and deaths in treated patients within the GSD are similar to observations in registry and survey datasets [18, 30, 38, 39, 65]. All cases had clinical courses consistent with COVID-19 as described in the general population. However, pharmacovigilance data are subject to multiple limitations. Because these data are spontaneously reported, details of patient characteristics, comorbidities, disease course, and outcomes are often limited and inconsistently supplied by healthcare providers. Limitations are accentuated by the high medical workload during the COVID-19 pandemic. Finally, selection bias may lead to under-reporting of mild cases, but more accurate reporting of moderate-to-severe cases. Therefore, the true incidence and/or severity of COVID-19 infection, or positive tests, cannot be known from these data. For this reason, we have not made conclusions regarding the comparative reporting rates of COVID-19 in this dataset. Nonetheless, there is currently no evidence to suggest an increased risk of COVID-19 infection in patients treated with these MS therapies.
For the IBM Explorys results, conclusions are limited by the low number of patients with COVID-19 in the MS cohort (n = 344), the lack of a healthy population comparator cohort, the retrospective nature of the study, and the large proportion of PwMS without an open DMT prescription (n = 123,185), potentially owing to incomplete data capture within the EHR database. Given that only ~ 20% of patients in the database had an open DMT prescription, it is likely that the “no open prescription” status reflects an incomplete data capture within this database. There also may have been patients in the database who were treated with an MS DMT without documentation in the EHR. Separately, decisions to treat with a DMT may be based on a perceived benefit-risk profile, leading to potential selection bias in those patients with an open DMT prescription. Thus, the resulting sample population may not be optimally representative of the prevalent MS population in the USA. Given the limited availability of COVID-19 testing in the first half of the data collection period, under-reporting of mild cases is likely during that timeframe. Asymptomatic COVID-19 cases are also likely to be missed in the IBM Explorys database or the Biogen GSD, resulting in potential misclassification bias. For example, if the frequency of develo** symptoms of COVID-19 is lower with IFNs or GA than with anti-CD20 treatment, patients treated with IFNs or GA will more often be under-reported.
While we acknowledge this analysis evaluated real-world data on patients receiving MS DMTs from two diverse databases, IBM Explorys and Biogen’s GSD, it is relevant to note that a profile emerges from the Biogen GSD regarding COVID-19 infection rates and clinical course in patients receiving IFNs, fumarates and natalizumab, which is consistent with both the real-world data from Explorys and with prior reports from other registry datasets. [18, 30, 38, 39, 65].
Disease-modifying therapies have different biological mechanisms, potentially explaining the differential risk and course of COVID-19 with different DMTs. Disease state (e.g., relapsing or progressive form of MS), disease severity, age, and level of disability may also contribute to variance in the rate of infection. Because EDSS data are absent from the EHR dataset, the impact of EDSS on the risk of infection is not evaluated. Furthermore, rates of infections follow risk behavior, including social distancing, mask wearing, occupation, and ventilation, as well as the COVID-19 prevalence in the local community.
With the availability of vaccines against SARS-CoV-2, the benefit vs risk for individual DMTs will be revisited. Antibody response to vaccines is reduced with anti-CD20s, alemtuzumab, and S1Ps [25]. However, there are adequate antibody responses to vaccines with anti-VLA-4 antibodies, IFNs, fumarates, and GA [25]. There is likely a mechanistic interaction between vaccine responses, MS immune disruption, and immune alterations from DMTs [25, 66]. Effects of DMTs on vaccine responses have implications for optimization of therapy and for public health, in MS and other chronic immune-mediated diseases.
5 Conclusions
Analyses of the IBM Explorys dataset and Biogen GSD expand and confirm findings from other real-world registries, and demonstrate that PwMS do not have a higher risk of infection with SARS-CoV-2. Comorbidities elevate the frequency of infection. Disease-modifying therapies also modify the risk of infection, likely dependent on their mechanisms of action. Patients with MS taking IFNs and GA had the lowest risk of COVID-19, and patients receiving anti-CD20 therapies had the highest risk. Despite the limitations of the data sources, these findings enhance strategic and personalized approaches to managing DMTs in MS during the ongoing COVID-19 pandemic.
References
World Health Organization. Pneumonia of unknown cause: China. 2020. https://www.who.int/csr/don/05-january-2020-pneumonia-of-unkown-cause-china/en/. Accessed 7 Sept 2020.
Fauci AS, Lane HC, Redfield RR. COVID-19: navigating the uncharted. N Engl J Med. 2020;382(13):1268–9.
Ge H, Wang X, Yuan X, **ao G, Wang C, Deng T, et al. The epidemiology and clinical information about COVID-19. Eur J Clin Microbiol Infect Dis. 2020;39(6):1011–9.
Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–74.
World Health Organization. WHO coronavirus disease (COVID-19) dashboard. 2020. https://covid19.who.int/?gclid=EAIaIQobChMIz-i4-f2i7AIVjIFaBR0ZXAxOEAAYASAAEgIiDPD_BwE. Accessed 7 Oct 2020.
Wei J, Zhao J, Han M, Meng F, Zhou J. SARS-CoV-2 infection in immunocompromised patients: humoral versus cell-mediated immunity. J Immunother Cancer. 2020;8(2):e000862.
Ciotti JR, Grebenciucova E, Moss BP, Newsome SD. Multiple sclerosis disease-modifying therapies in the COVID-19 era. Ann Neurol. 2020;88(6):1062–4. https://doi.org/10.1002/ana.25907.
Gianfrancesco M, Hyrich KL, Al-Adely S, Carmona L, Danila MI, Gossec L, et al. COVID-19 global rheumatology alliance. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2020;79(7):859–66.
Castelo-Branco A, Chiesa F, Conte S, Bengtsson C, Lee S, Minton N, et al. Infections in patients with multiple sclerosis: a national cohort study in Sweden. Mult Scler Relat Disord. 2020;45:102420.
Danza A, Ruiz-Irastorza G. Infection risk in systemic lupus erythematosus patients: susceptibility factors and preventive strategies. Lupus. 2013;22(12):1286–94.
Boziki MK, Mentis AFA, Shumilina M, Makshakov G, Evdoshenko E, Grigoriadis N. COVID-19 immunopathology and the central nervous system: implication for multiple sclerosis and other autoimmune diseases with associated demyelination. Brain Sci. 2020;10(6):345.
MS International Federation. Global COVID-19 advice for people with MS. 2020. http://www.msif.org/wp-content/uploads/2020/06/MSIF-Global-advice-on-COVID-19-for-people-with-MS-_-updated17June2020.pdf. Accessed 21 Oct 2020.
Bhatia R, Srivastava MVP, Khurana D, Pandit L, Mathew T, Gupta S, et al. Consensus statement on immune modulation in multiple sclerosis and related disorders during the COVID-19 pandemic: expert group on behalf of the Indian Academy of Neurology. Ann Indian Acad Neurol. 2020;23(Suppl. 1):S5-14.
Centers for Disease Control and Prevention. If you are immunocompromised, protect yourself from COVID-19. 2020. http://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/immunocompromised.html. Accessed 21 Oct 2020.
Rae-Grant A, Day GS, Marrie RA, Rabinstein A, Cree BAC, Gronseth GS, et al. Practice guideline recommendations summary: disease-modifying therapies for adults with multiple sclerosis: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2018;90(17):777–88.
Berger JR, Brandstadter R, Bar-Or A. COVID-19 and MS disease-modifying therapies. Neurol Neuroimmunol Neuroinflamm. 2020;7(4):e761.
Fan M, Qiu W, Bu B, Xu Y, Yang H, Huang D, et al. Risk of COVID-19 infection in MS and neuromyelitis optica spectrum disorders. Neurol Neuroimmunol Neuroinflamm. 2020;7(5):e787.
Parrotta E, Kister I, Charvet L, Sammarco C, Saha V, Charlson RE, et al. COVID-19 outcomes in MS: observational study of early experience from NYU Multiple Sclerosis Comprehensive Care Center. Neurol Neuroimmunol Neuroinflamm. 2020;7(5):e835.
Fernandez-Ruiz R, Masson M, Kim MY, Myers B, Haberman RH, Castillo R, et al. NYU WARCOV Investigators. Leveraging the United States epicenter to provide insights on COVID-19 in patients with systemic lupus erythematosus. Arthritis Rheumatol. 2020;72(12):1971–80.
Zen M, Fuzzi E, Astorri D, Saccon F, Padoan R, Ienna L, et al. SARS-CoV-2 infection in patients with autoimmune rheumatic diseases in northeast Italy: a cross-sectional study on 916 patients. J Autoimmun. 2020;112:102502.
Celius EG. Infections in patients with multiple sclerosis: implications for disease-modifying therapy. Acta Neurol Scand. 2017;136(Suppl. 201):34–6.
Sormani MP. Italian Study Group on COVID-19 infection in multiple sclerosis. An Italian programme for COVID-19 infection in multiple sclerosis. Lancet Neurol. 2020;19(6):481–2.
Giovannoni G, Hawkes C, Lechner-Scott J, Levy M, Waubant E, Gold J. The COVID-19 pandemic and the use of MS disease-modifying therapies. Mult Scler Relat Disord. 2020;39:102073.
Sallard E, Lescure FX, Yazdanpanah Y, Mentre F, Peiffer-Smadja N. Type 1 interferons as a potential treatment against COVID-19. Antivir Res. 2020;178:104791.
Ciotti JR, Valtcheva MV, Cross AH. Effects of MS disease-modifying therapies on responses to vaccinations: a review. Mult Scler Relat Disord. 2020;45:102439.
Louapre C, Collongues N, Stankoff B, Giannesini C, Papeix C, Bensa C, et al. Covisep Investigators. Clinical characteristics and outcomes in patients with coronavirus disease 2019 and multiple sclerosis. JAMA Neurol. 2020;77(9):1–10.
Tirupathi R, Muradova V, Shekhar R, Salim SA, Al-Tawfiq JA, Palabindala V. COVID-19 disparity among racial and ethnic minorities in the US: a cross sectional analysis. Travel Med Infect Dis. 2020;38:101904.
Bassett MT, Chen JT, Krieger N. Variation in racial/ethnic disparities in COVID-19 mortality by age in the United States: a cross-sectional study. PLoS Med. 2020;17(10):e1003402.
Zabalza A, Tagliani P, Cárdenas-Robledo S, Arrambide G, Otero-Romero S, Carbonell-Mirabent P, et al. COVID-19 in MS patients: susceptibility and severity risk factors. MSVirtual2020; ECTRIMS/ACTRIMS; 11–13 Sept 2020.
Klineova S, Harel A, Straus Farber R, Zhang Y, Deangelis T, Leung TM, et al. COVID-19 infection in patients with multiple sclerosis: an observational study by The New York COVID-19 Neuro-Immunology Consortium (NYCNIC). MSVirtual2020; ECTRIMS/ACTRIMS; 11-13 Sep 2020.
Loonstra FC, Hoitsma E, van Kempen ZL, Killestein J, Mostert JP. COVID-19 in multiple sclerosis: the Dutch experience. Mult Scler. 2020;26(10):1256–60.
Chaudhry F, Bulka H, Rathnam AS, Said OM, Lin J, Lorigan H, et al. COVID-19 in multiple sclerosis patients and risk factors for severe infection. J Neurol Sci. 2020;418:117147.
Sormani MP, De Rossi N, Schiavetti I, Carmisciano L, Cordioli C, Moiola L, et al. Musc-19 Study Group. Disease modifying therapies and COVID-19 severity in multiple sclerosis. Ann Neurol. 2021. https://doi.org/10.1002/ana.26028.
IBM. Explorys EHR solutions. https://www.ibm.com/products/explorys-ehr-data-analysis-tools. Accessed 9 Dec 2020.
Mathian A, Mahevas M, Rohmer J, Roumier M, Cohen-Aubart F, Amador-Borrero B, et al. Clinical course of coronavirus disease 2019 (COVID-19) in a series of 17 patients with systemic lupus erythematosus under long-term treatment with hydroxychloroquine. Ann Rheum Dis. 2020;79(6):837–9.
Najafi S, Rajaei E, Moallemian R, Nokhostin F. The potential similarities of COVID-19 and autoimmune disease pathogenesis and therapeutic options: new insights approach. Clin Rheumatol. 2020;39(11):3223–35.
International Conference on Harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonized tripartite guideline: guideline for good clinical practice. J Postgrad Med. 2001;47(1):45–50.
Simpson-Yap S, De Brouwer E, Kalincik T, Rijke N, Hillert J, Walton C, et al. First results of the COVID-19 in MS Global Data Sharing Initiative suggest anti-CD20 DMTs are associated with worse COVID-19 outcomes. MSVirtual2020; ECTRIMS/ACTRIMS; 11–13 Sept 2020.
Mendes MF, Ferreira MI, Sousa NA, Thomaz R, Apóstolos-Pereira SL, Alves-Leon S, et al. Incidence and clinical outcome of COVID-19 in a cohort of 11.560 Brazilian patients with multiple sclerosis. MSVirtual2020; ECTRIMS/ACTRIMS; 11–13 Sept 2020.
Winkelmann A, Loebermann M, Reisinger EC, Hartung HP, Zettl UK. Disease-modifying therapies and infectious risks in multiple sclerosis. Nat Rev Neurol. 2016;12(4):217–33.
Zheng C, Kar I, Chen CK, Sau C, Woodson S, Serra A, et al. Multiple sclerosis disease-modifying therapy and the COVID-19 pandemic: implications on the risk of infection and future vaccination. CNS Drugs. 2020;34(9):879–96.
Safavi F, Nourbakhsh B, Azimi AR. B-cell depleting therapies may affect susceptibility to acute respiratory illness among patients with multiple sclerosis during the early COVID-19 epidemic in Iran. Mult Scler Relat Disord. 2020;43:102195.
Simpson-Yap S, De Brouwer E, Kalincik T, Rijke N, Hillert J, Walton C, et al. Associations of DMT therapies with COVID-19 severity in multiple sclerosis. medRxiv. 2021. https://doi.org/10.1101/2021.02.08.21251316.
Fox RJ, Chan A, Gold R, Phillips JT, Selmaj K, Chang I, et al. Characterizing absolute lymphocyte count profiles in dimethyl fumarate-treated patients with MS: patient management considerations. Neurol Clin Pract. 2016;6(3):220–9.
Naismith RT, Wolinsky JS, Wundes A, LaGanke C, Arnold DL, Obradovic D, et al. Diroximel fumarate (DRF) in patients with relapsing-remitting multiple sclerosis: interim safety and efficacy results from the phase 3 EVOLVE-MS-1 study. Mult Scler. 2020;26(13):1729–39.
Longbrake EE, Mao-Draayer Y, Cascione M, Zielinski T, Bame E, Brassat D, et al. Dimethyl fumarate treatment shifts the immune environment toward an anti-inflammatory cell profile while maintaining protective humoral immunity. Mult Scler. 2020:1352458520937282.
von Hehn C, Howard J, Liu S, Meka V, Pultz J, Mehta D, et al. Immune response to vaccines is maintained in patients treated with dimethyl fumarate. Neurol Neuroimmunol Neuroinflamm. 2018;5(1):e409.
Mehta D, Miller C, Arnold DL, Bame E, Bar-Or A, Gold R, et al. Effect of dimethyl fumarate on lymphocytes in RRMS: Implications for clinical practice. Neurology. 2019;92(15):e1724–38.
Schweitzer F, Laurent S, Fink GR, Barnett MH, Hartung HP, Warnke C. Effects of disease-modifying therapy on peripheral leukocytes in patients with multiple sclerosis. J Neurol. 2020. https://doi.org/10.1007/s00415-019-09690-6.
Tallantyre EC, Whittam DH, Jolles S, Paling D, Constantinesecu C, Robertson NP, et al. Secondary antibody deficiency: a complication of anti-CD20 therapy for neuroinflammation. J Neurol. 2018;265(5):1115–22.
Reder A, Adamo A, Wicklein E-M, Bhatti A. Use and safety of interferon beta-1b during the COVID-19 outbreak: current data from a pharmacovigilance safety database. MSVirtual2020; ECTRIMS/ACTRIMS; 11–13 Sept 2020.
Zhang Q, Bastard P, Liu Z, Le Pen J, Moncada-Velez M, Chen J, et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. 2020;370(6515):eabd4570.
Pairo-Castineira E, Clohisey S, Klaric L, Bretherick A, Rawlik K, Parkinson N, et al. Genetic mechanisms of critical illness in COVID-19. MedRxiv. 2020. https://doi.org/10.1101/2020.09.24.20200048.
Bastard P, Rosen LB, Zhang Q, Michailidis E, Hoffmann HH, Zhang Y, et al. Auto-antibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370(6515):eabd4585.
Prod’homme T, Zamvil SS. The evolving mechanisms of action of glatiramer acetate. Cold Spring Harb Perspect Med. 2019;9(2):a029249.
Melnikov M, Sharanova S, Sviridova A, Rogovskii V, Murugina N, Nikolaeva A, et al. The influence of glatiramer acetate on Th17-immune response in multiple sclerosis. PLoS ONE. 2020;15(10):e0240305.
Hausler D, Hajiyeva Z, Traub JW, Zamvil SS, Lalive PH, Bruck W, et al. Glatiramer acetate immune modulates B-cell antigen presentation in treatment of MS. Neurol Neuroimmunol Neuroinflamm. 2020;7(3):e698.
Rommer PS, Milo R, Han MH, Satyanarayan S, Sellner J, Hauer L, et al. Immunological aspects of approved MS therapeutics. Front Immunol. 2019;10:1564.
Bar-Or A, Calkwood JC, Chognot C, Evershed J, Fox EJ, Herman A, et al. Effect of ocrelizumab on vaccine responses in patients with multiple sclerosis: the VELOCE study. Neurology. 2020;95(14):e1999-2008.
Pescovitz MD, Torgerson TR, Ochs HD, Ocheltree E, McGee P, Krause-Steinrauf H, et al. Effect of rituximab on human in vivo antibody immune responses. J Allergy Clin Immunol. 2011;128(6):1295-302.e5.
Genentech. Ocrevus highlights of prescribing information. 2020. https://www.gene.com/download/pdf/ocrevus_prescribing.pdf. Accessed 19 Jan 2021.
Mateen FJ, Rezaei S, Alakel N, Gazdag B, Kumar AR, Vogel A. Impact of COVID-19 on U.S. and Canadian neurologists' therapeutic approach to multiple sclerosis: a survey of knowledge, attitudes, and practices. J Neurol. 2020;267(12):3467–75.
National Multiple Sclerosis Society. MS treatment guidelines during the coronavirus pandemic. https://www.nationalmssociety.org/coronavirus-covid-19-information/multiple-sclerosis-and-coronavirus/ms-treatment-guidelines-during-coronavirus. Accessed 8 Feb 2021.
MS-UK. MS and COVID-19. 2021. https://www.ms-uk.org/ms-and-coronavirus. Accessed 25 Jan 2021.
Hughes R, Pedotti R, Koendgen H. COVID-19 in persons with multiple sclerosis treated with ocrelizumab – a pharmacovigilance case series. Mult Scler Relat Disord. 2020;42:102192.
Sadeghmousavi S, Rezaei N. COVID-19 and multiple sclerosis: predisposition and precautions in treatment. SN Compr Clin Med. 2020. https://doi.org/10.1007/s42399-020-00504-9.
Acknowledgements
Biogen provided funding for medical writing support in the development of this manuscript; Karen Spach, PhD, from Excel Scientific Solutions wrote sections of the first draft of the manuscript based on extensive input from authors, and Nathaniel Hoover from Excel Scientific Solutions copy edited and styled the manuscript as per journal requirements. The authors gratefully acknowledge the contribution of Bernd Kieseier during the early stages of this work. The authors reviewed and revised the manuscript and had full editorial control of the manuscript and provided their final approval of all content.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Biogen providing funding for the study.
Conflict of interest
Anthony T. Reder has received unrestricted research support from Bayer, Biogen, Roche-Genentech, Mallinckrodt, Merck-Serono, and Novartis, and is a consultant for Bayer, Biogen, NKMax America, Merck-Serono, Novartis, and Roche-Genentech. Diego Centonze is an advisory board member of Almirall, Celgene, BMS, Bayer Schering, Biogen, GW Pharmaceuticals, Merck Serono, Novartis, Roche, Sanofi-Genzyme, and Teva and has received honoraria for speaking or consultation fees from Almirall, Bayer Schering, Biogen, GW Pharmaceuticals, Merck Serono, Novartis, Roche, Sanofi-Genzyme, and Teva. He is also the principal investigator in clinical trials for Abbvie, Zambon, Bayer Schering, Biogen, Merck Serono, Mitsubishi, Novartis, Roche, Sanofi-Genzyme, and Teva. His preclinical and clinical research was supported by grants from Bayer Schering, Biogen, Celgene, Lundbeck, Merck Serono, Novartis, Roche, Sanofi-Genzyme, and Teva. Maria L. Naylor, Anjali Nagpal, Rajani Rajbhandari, Arman Altincatal, Michelle Kim, Aaron Berdofe, Maha Radhakrishnan, Eunice Jung, Alfred W. Sandrock, Karen Smirnakis, Catrinel Popescu, and Carl de Moor are employees and hold stock/stock options in Biogen.
Ethics approval
Not applicable.
Consent to participate
The data extracted from the databases were entirely anonymized and therefore do not require informed consent.
Consent for publication
Not applicable.
Availability of data and material
The datasets generated during and/or analyzed during the current study are not publicly available because Biogen does not own the data and it would be difficult to make this data source public, but the portions that are publicly available will be provided by the corresponding author on reasonable request.
Code availability
Not applicable.
Author contributions
All authors have read and approved the final manuscript. ATR provided writing, data analysis, and the scientific basis of the text. DC provided discussion on methods of the study and critical reading of the manuscript. MLN and AN provided the scientific basis of the text, data analysis and interpretation, and writing. RR and AA provided data analysis and interpretation. MK and AB provided data management and informatics support. MR provided the scientific basis of the text and interpretation. EJ provided electronic health record subject matter expert guidance, and data management and informatics support. AWS provided the scientific basis of the text and interpretation. KS and CP provided expert information from the Biogen Global Safety Database. CdM provided data analysis and interpretation.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Reder, A.T., Centonze, D., Naylor, M.L. et al. COVID-19 in Patients with Multiple Sclerosis: Associations with Disease-Modifying Therapies. CNS Drugs 35, 317–330 (2021). https://doi.org/10.1007/s40263-021-00804-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-021-00804-1