Summary
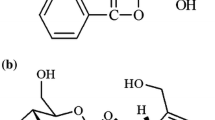
The objective of the present study was to investigate the in vivo pharmacokinetics of huperzine A in healthy human volunteers. Twelve subjects (M 6, F 6; age ranged from 20 ∼ 25 years) participated in the study. Huperzine A was administered in tablet form at a single dose of 0.4 mg. Following oral administration, the presence of huperzine A started to appear in the plasma at 5—10 min, and reached the peak concentrations with a Cmax of 2.59±0.37 ng/ml at 58.33±3.89 min (time to reach peak level, Tmax). The area under plasma vs time curve (AUC0−t) and the area under plasma from zero to infinity (AUC0−∞) for huperzine A were found to be 1986.96± 164.57 μg/1-min and 2450.34±233.32 μg/1-min, respectively. The results of this study indicated that the pharmacokinetics of huperzine A conformed to a two-compartmental open model. The mean values of α and the β half-life were 21.13±7.28 min and 716.25±130.18 min respectively, and snowed a biphasic profile with rapid distribution followed by a slower elimination rate.
Similar content being viewed by others
References
Liu J.S., Zhu Y.L., Zhou Y.Z., Han Y.Y., Wu F.W., Qi B.F., (1986): The structure of huperzine A and B, two new alkaloids exhibiting marked anticholinesterase activity. Can. J. Chem. 64, 837–839.
Tang X.C., Han Y.F., Chen X.P., Zhu X.D., (1986): Effects of huperzine A on learning and retrieval processes of discrimination performance in rats. Acta Pharmacol. Sin. 7, 507–511.
Tang X.C., De S.P., Sugaya K., Giacobini E., (1989): Effect of huperzine A, a new Cholinesterase inhibitor, on the central cholinergic system of the rat. J. Neur. Res. 24, 276–285.
Tang X.C., (1996): Huperzine A (Shuangyi**): a promising drug for Alzhemimer’s disease. Zhongguo Yaoli Xuebao 17, 481–484.
Zangara A., (2003): The psychopharmacology of huperzine A: an alkaloid with cognitive enhancing and neuroprotective properties of interest in the treatment of Alzheimer’s disease. Pharmacol. Biochem. Behav. 75, 675–686.
Qian B.C., Wang M., Zhou Z.F., (1995): Pharmacokinetics of tablet huperzine A in six volunteers. Acta Pharmacol. Sin. 16, 396–398.
Wang Y.E., Feng J., Lu W.H., (1988): Pharmaceutics of huperaine A in rat and mice. Acta Pharmacol. Sin. 9, 193–197.
Wang Y.W., Chu D.F., Gu J.K., (2004): Liquid chromatographic tandem mass spectrometric method for the quantitation of huperzine A in dog plasma. J. Chromatogr. B. 4, 375–378.
Yue P., Zhao Y., Tao T., (2005): Determination of hupezine A in cerebrospinal fluid of rats by HPLC-Fluorescence. Zhongguo Yaoxue Zazhi. 40, 1503–1507.
Li C, Du F.F., Yu C, (2004): A sensitive method for the determination of the novelcholinesterase inhibitor ZT-1 and its active metabolite huperzine A in rat blood using liquid chromatography /tandem mass spectrometry, Rapid Commun. Mass Spectrom. 18, 651–656.
Li Y.X., Jiang X.H., Lan K., Wang L., (2007): Simple determination of huperzine A in human plasma by liquid chromatographic tandem mass spectrometric method, Biomed. Chromatogr. 21, 15–20.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Li, Y.X., Zhang, R.Q., Li, C.R. et al. Pharmacokinetics of huperzine A following oral administration to human volunteers. Eur. J. Drug Metabol. Pharmacokinet. 32, 183–187 (2007). https://doi.org/10.1007/BF03191002
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF03191002