Summary
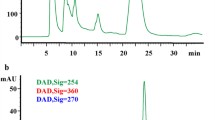
Ten adult volunteers with an average age 28 years were given a single oral dose of six tablets of Ginkgo biloba extract. Quercetin and kaempferol in different period of human urine were determined by using RP-HPLC.
The results showed the elimination rate constantk and the absorption rate constantk a of quercetin were slightly more than that of kaempferol; and the absorption half-life (t1/2a), the elimination half-life (t1/2) and tmax of qurcetin were less than that of kaempferol, the differences were, however, not statistically significant.
The mean values ofk a were 0.61 h−1 and 0.55 h−1, t1/2a 1.51h and 1.56h,k 0.37 h−1 and 0.30 h−1, t1/2 2.17h and 2.76h, Tmax 2.30h and 2.68h for quercetin and kaempferol, respectively, which mean absorption and elimination of quercetin and kaemperferol are 0.17% and 0.22%, respectively.
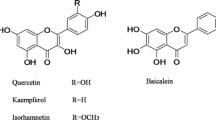
Quercetin and kaemferol are excreted in the human urine mainly as glucuronides.
Similar content being viewed by others
References
Hollman, PC; de-Vries, JH; van-Leeuwen, SD; Mengelers, MJ; Katan, MB. (1995) Absorption of dietary quercetin glycosides and quercetin in healthy ileostomy volunteers. Am. J. Clin. Nutr. 62(6): 1276–82
Hollman, PC. Ph. D. Thesis, (1997) State Institute for Quality Control of Agricultural Products (RIKILT-DLO). Wageningen. Netherlands.
Nielsen, SE; Kall, M; Justesen, U; Schou, A; Dragsted, LO. (1997) Human absorption and excretion of flavonoids after broccoli consumption. Cancer-Lett. 114(1–2): 173–4.
Wojcicki J, Gawronska-Szklarz B, Bieganowski W, Patalan M, Smulski HK, Samochowiec L, Zakrzewski J. Comparative pharmacokinetics and bioavailability of flavonoid glycosides of Ginkgo biloba after a single oral administration of three formulations to healthy volunteers. Mater Med Pol, 1995;27(4):141–6
Hollman, -P-C; van-Trijp, -J-M; Buysman, -M-N; van-der-Gaag, -M-S; Mengelers, -M.-J; de-Vries, -J-H; Katan, -M-B. (1997) Relative bioavailability of the antioxidant flavonoid quercetin from various foods in man. FEBS-Lett. 418(1–2): 152–6.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wang, F.M., Yao, T.W. & Zeng, S. Disposition of quercetin and kaempferol in human following an oral administration of Ginkgo biloba extract tablets. Eur. J. Drug Metab. Pharmacokinet. 28, 173–177 (2003). https://doi.org/10.1007/BF03190482
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF03190482