Abstract
According to the oxidative hypothesis of atherosclerosis, a hyperoxidizability of lipoproteins could favor the development of the atherosclerotic process. Besides, it has been recently reported that models of elevated very-low-density-lipoprotein (VLDL) levels in rats resulted in an increased susceptibility of these VLDL to oxidation. Treatment with dexamethasone classically induces an increase in plasma VLDL concentration. The aim of our study was thus to assess the effects of a treatment with dexamethasone in rats on the susceptibility to copper oxidation, both on total plasma and on isolated lipoproteins.
Male Sprague-Dawley rats aged three months were treated with a daily intraperitoneal injection of dexamethasone (1.5 mg per kg) for five days (DEX group), whereas control rats were fedad libitum (AL group). In order to take into account the decrease of food intake induced by dexamethasone treatment, a group ofpair-fed rats was constituted (PF group). These rats had the same food intake as rats of the DEX group and were treated with a daily isovolumic intraperitoneal injection of NaCl for 5 d. After 5 d treatment, rats were fasted overnight, then killed, and blood was collected on EDTA. Low-density lipoproteins (VLDL+LDL) and high-density lipoproteins (HDL) were isolated by ultracentrifugation. A copper oxidation was conducted both on total plasma and on isolated lipoproteins.
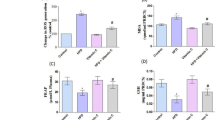
As expected, after treatment with dexamethasone, plasma exhibited increased triglyceride and glucose levels. Similarly, VLDL + LDL of rats from the DEX group were enriched with triglycerides, when compared with VLDL + LDL of the other two groups of rats. Our major finding was a marked increase in the susceptibility of total plasma of the DEX group to copper oxidation, in comparison with the other two groups of rats. This oxidizability was assessed by the maximal level of oxidation products absorbing at 234 nm and classically considered to be conjugated dienes (7.46±0.70 μmol L−1 in the DEX group vs 3.36±0.40 and 2.05 ±0.60 μmol L−1 in the AL and PF groups, respectively). Nevertheless, this higher oxidizability was not observed in the isolated lipoprotein fractions, as shown by the formation of lipid peroxidation products such as conjugated dienes, thiobarbituric-acid reactive substances, hydroperoxides, 7-ketocholesterol, and dienals. This is not in agreement with other models of hypertriglyceridemia that have been reported to induce a hyperoxidizability of lipoproteins in rats. Our results led us to hypothesize that other plasma components such as proteins could be involved in this susceptibility to oxidation. Indeed, the severe protein catabolism induced by dexamethasone treatment could support this hypothesis, by forming protein components that are more susceptible to oxidation, as shown by an increased carbonyl formation upon plasma copper oxidation.
Similar content being viewed by others
Abbreviations
- d:
-
density
- HDL:
-
high density lipoprotein
- LDL:
-
low density lipoprotein
- mRNA:
-
messenger ribonucleic acid
- Na2EDTA:
-
disodium ethylene diamine tetraacetate
- TBARS:
-
thiobarbituric acid-reactive substances
- VLDL:
-
very low density lipoprotein
References
Taskinen, M. R., Nikkilä, E. A., Pelkonen, R., and Sane, T. (1983).J. Clin. Endocrinol. Metab. 57, 619–626.
Mori, W., Aoyama, H., and Mori, N. (1984).Jpn. J. Exp. Med. 54, 255–261.
Asai, K., Funaki, C., Hayashi, T., Yamada, K., Naito, M., Kuzuya, M., Yoshida, F., Yoshimine, N., and Kuzuya, F. (1993).Arterioscl. Thromb. 13, 892–899.
Cole, T. G., Wilcox, H. G., and Heimberg, M. (1982).J. Lipid Res. 23, 81–91.
Martin-Sanz, P., Vance, J. E., and Brindley, D. N. (1990).Biochem. J. 271, 575–583.
Reaven, E. P., Kolterman, O. G., and Reaven, G. M. (1974).J. Lipid Res. 15, 74–83.
Rayssiguier, Y., Gueux, E., Bussière, L., and Mazur, A. (1993).J. Nutr. 123, 1343–1348.
Garfield, S. A., Scott, A. C., and Cardell, R. R. (1978).Anat. Rec. 192, 73–78.
Bagdade, J. D., Yee, E., Albers, J., and Pykalisto, O. J. (1976).Metabolism 25, 533–542.
Steinberg, D., Parthasarathy, S., Carew, T. E., Khoo, J. C., and Witztum, J. L. (1989).New Engl. J. Med. 320, 915–924.
Gueux, E., Cubizolles, C., Bussière, L., Mazur, A., and Rayssiguier, Y. (1993).Lipids 28, 573–575.
Mazur, A., Gueux, E., Bureau, I., Feillet-Coudray, C., Rock, E., and Rayssiguier, Y. (1998).Atherosclerosis 137, 443–445.
Hicks, J. J., Silva-Gomez, A. B., and Vilar-Rojas, C. (1997).Life Science 60, 2059–2067.
Yamada, K., Naito, M., Hayashi, T., Asai, K., Yoshimine, N., and Igushi, A. (1993).Artery 20, 253–267.
Bulkley, B. H., and Roberts, W. C. (1975).Am. J. Med. 58, 243–264.
Kalbak, K. (1972),Ann. Rheum. Dis. 31, 196–200.
Nashel, D. J. (1986).Am. J. Med. 80, 925–929.
Esterbauer, H., Striegl, G., Puhl, H., and Rotheneder, M. (1989).Free Rad. Res. Commun. 6, 67–75.
Levine, R. L., Garland, D., Oliver, C. N., Amici, A., Climent, I., Lenz, A. G., Ahn, B. W., Shaltiel, S., and Stadman, E. R. (1990).Methods Enzymol. 186, 464–478.
Brindly, D. N. (1981).Clin. Sci. 61, 129–133.
Stern, M. P., Kolterman, O. G., Fries, J. F., McDevitt, H. O., and Reaven, G. M. (1973).Arch. Intern. Med. 132, 97–101.
El-Shaboury, A. H. and Hayes, T. M. (1973).Br. Med. J. 2, 85–86.
Wang, C. H., McLeod, R. S., Yao, Z., and Brindley, D. N. (1995).Arterioscler. Thromb. Vasc. Biol. 15, 1481–1491.
Ong, J. M., Simsolo, R. B., Saffari, B., and Kern, P. A. (1992).Endocrinology 130, 2310–2316.
Minet-Quinard, R., Moinard, C., Villié, F., Walrand, S., Vasson, M. P., Chopineau, J., and Cynober, L. (1999).Am. J. Physiol. 226, E558-E564.
Lyons, T. J. (1991).Diabetic Med. 8, 411–419.
Scaccini, C., Nardini, M., D'Aquino, M., Gentili, V., DiFelice, M., and Tomassi, G. (1992).J. Lipid Res. 33, 627–633.
Feurgard, C., Bayle, D., Guézingar, F., Sérougne, C., Mazur, A., Lutton, C., Aigueperse, J., Gourmelon, P., and Mathé, D. (1998).Radiat. Res. 150, 43–51.
Simpson, K. L. (1983).Proc. Nutr. Soc. 42, 7–17.
Kang, M. Y., Tsuchiya, M., Packer, L., and Manabe, M. (1998).Acta Anaesthesiol. Scand. 42, 4–12.
Taniguchi, S., Yanase, T., Kobayashi, K., Takayanagi, R., Haji, M., Umeda, F., and Nawata, H. (1994).Endocr. J. 41, 605–611.
Morel, D. W., and Chisolm, G. M. (1989).J. Lipid Res. 30, 1827–1834.
Stadtman, E. R. (1993).Annu. Rev. Biochem. 62, 797–821.
Max, S. R., Mill, J., Mearow, K., Konagaya, M., Konagaya, Y., Thomas, J. W., Banner, C., and Vitkovic, L. (1988).Am. J. Physiol. 255, E397-E403.
Ardawi, M. S., and Jamal, Y. S. (1990).Clin. Sci. Mol. Med. 79, 139–147.
De Vos, P., Saladin, R., Auwerx, J., and Staels, B. (1995).J. Biol. Chem. 270, 15958–15961.
Di Silvestro, R. A. and Jones, A. A. (1996).Biochim. Biophys. Acta 1317, 81–83.
Allain, C. C., Poon, L. S., Chan, C., Richmond, S. G., and Fu, P. C. (1974).Clin. Chem. 20, 470–475.
Eggstein, M., and Kreutz, F. H. (1966).Klin. Wochenschr. 44, 262–267.
Takayama, M., Itoh, S., Nagasaki, T., and Tanimipu, J. (1977).Clin. Chim. Acta 79, 93–98.
Yagi, K. (1976).Biochem. Med. 15, 212–216.
Arnaud, J., Fortis, I., Blachier, S., Kia, D., and Favier, A. (1991).J. Chromatogr. 572, 103–116.
Spranger, T., Finckh, B., Fingerhut, R., Kohlschütter, A., Beisigel, U., and Kontush, A. (1998).Chem. Phys. Lipids 91, 39–52.
Chapman, M. J. (1980).J. Lipid Res. 21, 789–853.
Di Silvestro, R. A. and Blostein-Fuji, A. (1997).Free Radic. Biol. Med. 22, 739–742.
Watanabe, N., Kamei, S., Ohkubo, A., Yamanaka, M., Ohsawa, S., Makino, K., and Tokuda, K. (1986).Clin. Chem. 32, 1551–1554.
Pinchuk, I., and Lichtenberg, D. (1996).Free Radic. Res. 24, 351–360.
Conover, W. J. (1980).Practical nonparametric statistics. Wiley: New York.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Belkebir-Mesbah, D., Bonnefont-Rousselot, D., Frey-Fressart, V. et al. Consequences of treatment with dexamethasone in rats on the susceptibility of total plasma and isolated lipoprotein fractions to copper oxidation. Endocr 10, 233–242 (1999). https://doi.org/10.1007/BF02738622
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02738622