Abstract
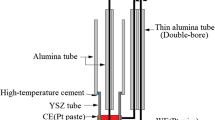
The equilibrium partial pressure of oxygen for a system containing coexisting NiCr2O4, Cr2O3, and Ni was determined at 1000 to 1300 K using the electrochemical technique. Yttria-stabilized zirconia was employed as a solid-state electrolyte in the open-circuit electromotive force (EMF) measurements. Results were compared to the reported data in the literature. The standard Gibbs energy of NiCr2O4 at 1000 to 1300 K was determined to be ΔG°f(NiCr2O4)=-1327.2+0.314T±2.874 (kJ/mole)
Similar content being viewed by others
References
J.D. Tretjakow and H. Schmalzried:Ber. Bunsen-Ges Phys. Chem., 1965, vol. 69, pp. 396–402.
V.A. Levitskii, T.N. Rezukhina, and V.G. Dneprova:Elektrokhimiya, 1965, vol. 1 (8), pp. 933–40.
A. Kozlowska-Rog and G. Rog:Polym. J. Chem., 1973, vol. 47, pp. 869–70.
W. Kunnmann, D.B. Rogers, and A. Wold:J. Phys. Chem. Solids, 1963, vol. 24, pp. 1535–38.
F. Muller and J. Kleppa:J. Inorg. Nucl. Chem., 1973, vol. 35, pp. 2673–78.
H. Davies and W.W. Smeltzer:J. Electrochem. Soc., 1974, vol. 121 (4), pp. 543–49.
S.C. Schaefer: RI No. 9043, U.S. Bureau of Mines, Albany, OR, 1986.
B.C.H. Steele:Electromotive Force Measurements in High Temperature Systems, C.B. Alcock, ed., Elsevier Science Publishing Co., Inc., New York, NY, 1968, pp. 3–28.
K. Kiukkola and C. Wagner:J. Electrochem. Soc., 1957, vol. 104, pp. 379–87.
R.A. Rapp and D.A. Shores: inTechniques of Metals Research, R.A. Rapp, ed., Interscience Publishers, New York, NY, 1970, vol. IV, part 2, p. 123.
R.A. Rapp:Trans. A1ME, 1963, vol. 227, pp. 371–74.
B.C.H. Steele and C.B. Alcock:Trans. AIME, 1965, vol. 233, pp. 1359–67.
J. Elliott and M. Gleiser:Thermochemistry for Steelmaking, Addison-Wesley Publishing Co., Inc., Reading, MA, 1960, vol. 1.
H.M. Chen and J. Chipman:Trans. ASM, 1947, vol. 38, p. 70.
J.P. Coughlin:Contributions to the Data on Theoretical Metallurgy, U.S. Bureau of Mines Bull. 542, U.S. Government Printing Office, Washington, DC, 1954.
Y. Jeannin, C. Mannerskantz, and F.D. Richardson:Trans. AIME, 1963, vol. 227, p. 300.
O. Kubaschewski, E.L. Evans, and C.B. Alcock:Metallurgical Thermochemistry, Pergamon Press, Inc., Elmsford, NY, 1967.
L.A. Pugliese and G.R. Fitterer:Metall. Trans., 1970, vol. 1, pp. 1997–2002.
F.N. Mazandarany and R.D. Pehlke:J. Electrochem. Soc., 1974, vol. 121, p. 711.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kung, SC. Gibbs energy of formation of nickel chromite. Metall Trans B 22, 673–675 (1991). https://doi.org/10.1007/BF02679023
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02679023