Abstract
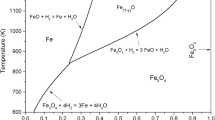
The internal reduction of high-grade granular hematite ore in hydrogen and carbon monoxide, and also the internal oxidation of porous iron granules in CO2-CO mixtures have been investigated. To assist the interpretation of the rate data for porous iron and iron oxides, rate measurements have been made also with dense wustite, previously grown on iron by oxidation. The iron formed by reduction of dense wustite is porous, similar to that observed when porous hematite is reduced. It is found that the rate of dissociation or formation of water vapor or carbon dioxide on the iron surface is about an order of magnitude greater than that on the surface of wustite. The results of the previous investigations using dense iron and wustite are in general accord with the present findings. The rate of reduction of hematite increases with increasing pore surface area of the reduced oxide. The results indicate that the rate of reduction of granules is controlled primarily by the formation of H2O or CO2 on the pore walls of wustite. The specific rate constants evaluated from internal reduction, using the total pore surface area, are about 1/50 to 1/100 of those for dense wustite. These findings indicate that with porous wustite or iron, the effective pore surface area utilized is about 1 to 2 pct of the total pore surface area. The rate of reduction in H2-CO mixtures is in accord with that derived from the rate constants for reduction in H2 and CO.
Similar content being viewed by others
References
E. T. Turkdogan and J. V. Vinters:Met. Trans., 1971, vol. 2, pp. 3175–88.
E. T. Turkdogan, R. G. Olsson, and J. V. Vinters:Met. Trans., 1971, vol. 2, pp. 3189–96.
W. M. McKewan and B. B. Rice: private communication, Fundamental Res. Lab. U. S. Steel Corporation, Monroeville, Pa.
L. Himmel, R. F. Mehl, and C. E. Birchenall:AIME Trans., 1953, vol. 197, pp. 827–43.
P. Hembree and J. B. Wagner:Trans. TMS-AIME, 1969, vol. 245, pp. 1547–52.
O. H. Gellner and F. D. Richardson:Nature, 1951, vol. 168, pp. 23–24.
K. Hauffe and H. Z. Pfeiffer:Z. Metallic., 1953, vol. 44, pp. 27–36.
F. S. Pettit and J. B. Wagner:Acta Met., 1964, vol. 12, pp. 35–40.
W. W. Smeltzer, L. A. Morris, and R. C. Logani:Can. Met. Quart., 1970, vol. 9, pp. 513–19.
H.-J. Grabke:Z. Elektrochem., 1965, vol. 69, pp. 48–57.
H.-J. Grabke:Ber. Bunsenges. Phys. Chem., 1967, vol. 71, pp. 1067–73.
L. S. Darken and E. T. Turkdogan:Heterogeneous Kinetics at Elevated Temperatures, pp. 25–92, Plenum Press, 1970.
H.-J. Grabke: Proc. 3rd Inter. Congress on Catalysis, pp. 928–38, North-Holland Pub. Co., Amsterdam, 1965.
R. J. Fruehan and L. J. Martonik:High Temp. Sci., 1971, vol. 3, no. 3, pp. 244–56.
E. T. Turkdogan and P. Grieveson:J. Electrochem. Soc., 1967, vol. 114, pp. 59–64.
E. T. Turkdogan, W. M. McKewan, and L. Zwell:J. Phys. Chem., 1965, vol. 69, pp. 327–34.
W. A. Edminston and R. E. Grace:Trans. TMS-AIME, 1966, vol. 236, pp. 1547–50.
J. H. Swisher and E. T. Turkdogan:Trans. TMS-AIME, 1967, vol. 239, pp. 426–31.
E. T. Turkdogan, R. G. Olsson, and J. V. Vinters:Carbon, 1970, vol. 8, pp. 545–64.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Turkdogan, E.T., Vinters, J.V. Gaseous reduction of iron oxides: Part III. Reduction-oxidation of porous and dense iron oxides and iron. Metall Trans 3, 1561–1574 (1972). https://doi.org/10.1007/BF02643047
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02643047