Abstract
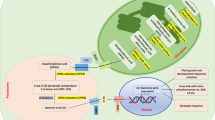
In higher plants, the membrane lipids contain a high proportion of trienoic fatty acids. It has been suggested that these fatty acids, especially linolenic acid, play an important role as a precursor to a defense-related signal molecule, jasmonate. In Arabidopsis, three genes encoding the ε-3 fatty acid desaturase, namely, FAD3, FAD7 and FAD8, are responsible for the production of trienoic fatty acids. TheFAD3 enzyme is localized in microsomes, while theFAD7 and theFAD8 enzymes are localized in plastid membranes. Environmental stimuli, such as wounding, salt stress and pathogen invasion, which lead to a rapid increase in jasmonate production, significantly induce expression of theFAD7 andFAD8 genes. Recent findings have supported the view that plastids are involved in jasmonate production. We have been able to clarify the regulatory mechanism of a plastid ε-3 desaturase gene by analyzing the ArabidopsisFAD7 promoter with respect to tissue-specific, light-responsive and wound-induced expression. In particular, this promoter provides a unique model for studying the mechanism of transcriptional activation through wound signal transduction pathways.
Similar content being viewed by others
References
Arondel, V., Lemieux, B., Hwang, I., Gibson, S., Goodman, H.M. andSomerville, C.R. 1992. Map-based cloning of a gene controlling omega-3 fatty acid desaturation inArabidopsis. Science258: 1353–1355.
Berberich, T., Harada, M., Sugawara, K., Kodama, H., Iba, K. andKusano, T. 1998. Two maize genes encoding ω-3 fatty acid desaturase and their differential expression to temperature. Plant Mol Biol.36: 297–306.
Blée, E. andJoyard, J. 1996. Envelope membranes from spinach chloroplasts are a site of metabolism of fatty acid hydroperoxides. Plant Physiol.110: 445–454.
Bowsher, C.G., Ferrie, B.J., Ghosh, S., Todd, J., Thompson, J.E. andRothstein, S.J. 1992. Purification and partial characterization of a membrane-associated lipoxygenase in tomato fruit. Plant Physiol.100: 1802–1807.
Browse, J., McCourt, P. andSomerville, C. 1986. A mutant of Arabidopsis deficient in C18∶3 and C16∶3 leaf lipids. Plant Physiol.81: 859–864.
Buell, C.R. andSomerville, C. 1995. Expression of defense-related and putative signaling genes during tolerant and susceptible interactions of Arabidopsis withXanthomonas campestris pv.campestris. Mol. Plant-Microbe Interact.8: 435–443.
Creelman, R.A. andMullet, J.E. 1995. Jasmonic acid distribution and action in plants: Regulation during development and response to biotic and abiotic stress. Proc. Natl. Acad. Sci. USA92: 4114–4119.
Creelman, R.A., Tierney, M.L. andMullet, J.E. 1992. Jasmonic acid/methyl jasmonate accumulate in wounded soybean hypocotyls and modulated wound gene expression. Proc. Natl. Acad. Sci. USA89: 4938–4941.
Farmer, E.E. 1994. Fatty acid signaling in plants and their associated microorganisms. Plant Mol. Biol.26: 1423–1437.
Farmer, E.E. andRyan, C.A. 1992. Octadecanoid precursors of jasmonic acid activate the synthesis of wound-inducible proteinase inhibitors. Plant Cell4: 129–134.
Gibson, S., Arondel, V., Iba, K. andSomerville, C. 1994. Cloning of a temperature-regulated gene encoding a chloroplast ω-3 desaturase fromArabidopsis thaliana. Plant Physiol.106: 1615–1621.
Gilmartin, P.M., Sarokin, L, Memelink, J. andChua, N.-H. 1990. Molecular light switches for plant genes. Plant Cell2: 369–378.
Goldsbrough, A.P., Albrecht, H. andStratford, R. 1993. Salicylic acid-inducible binding of a tobacco nuclear protein to a 10 bp sequence which is highly conserved amongst stress-inducible genes. Plant J.3: 563–571.
Hamada, T., Kodama, H., Nishimura, M. andIba, K. 1994. Cloning of a cDNA encoding tobacco ω-3 fatty acid desaturase. Gene147: 293–294.
Hamada, T., Nishiuchi, T., Kodama, H., Nishimura, M. andIba, K. 1996. cDNA cloning of a wounding-inducible gene encoding a plastid ω-3 fatty acid desaturase from tobacco. Plant Cell Physiol.37: 606–611.
Horiguchi, G., Kawakami, N., Kusumi, K., Kodama, K. andIba, K. 1998. Developmental regulation of genes for microsome and plastid ω-3 fatty acid desaturases in wheat (Triticum aestivum L.). Plant Cell Physiol.39: 540–544.
Iba, K., Gibson, S., Nishiuchi, T., Fuse, T., Nishimura, M., Arondel, V., Hugly, S. andSomerville, C. 1993. A gene encoding a chloroplast ω-3 fatty acid desaturase complements alterations in fatty acid desaturation and chloroplast copy number of the fad7 mutant ofArabidopsis thaliana. J. Biol. Chem.268: 24099–24105.
Kirsch, C., Takamiya-Wik, M., Reinold, S., Hahlbrock, K. andSomssich, I.E. 1997. Rapid, transient, and highly localized induction of the plastidial ω-3 fatty acid desaturase mRNA at fungal infection sites inPetroselinum crispum. Proc. Natl. Acad. Sci. USA94: 2079–2084.
Kodama, H., Hamada, T., Horiguchi, G., Nishimura, M. andIba, K. 1994. Genetic enhancement of cold tolerance by expression of a gene for chloroplast ω-3 fatty acid desaturase in transgenic tobacco. Plant Physiol.105: 601–605.
Kodama, H., Horiguchi, G., Nishiuchi, T., Nishimura, M. andIba, K. 1995. Fatty acid desaturation during chilling acclimation is one of the factors involved in conferring low-temperature tolerance to young tobacco leaves. Plant Physiol.107: 1177–1185.
Kodama, H., Akagi, H., Kusumi, K., Fujimura, T. andIba, K. 1997. Structure, chromosomal location and expression of a rice gene encoding the microsome ω-3 fatty acid desaturase. Plant Mol. Biol.33: 493–502.
Lemieux, B., Miquel, M., Somerville, C. andBrowse, J. 1990. Mutants ofArabidopsis with alterations in seed lipid fatty acid composition. Theor. Appl. Genet.80: 234–240.
McConn, M. andBrowse, J. 1996. The critical requirement for linolenic acid is pollen development, not photosynthesis, in an Arabidopsis mutant. Plant Cell8: 403–416.
McConn, M., Hugly, S., Browse, J. andSomerville, C. 1994. A mutation at the fad8 locus of Arabidopsis identifies a second chloroplast ω-3 desaturase. Plant Physiol.106: 1609–1614.
McConn, M., Creelman, R.A., Bell, E., Mullet, J.E. andBrowse, J. 1997. Jasmonate is essential for insect defense inArabidopsis. Proc. Natl. Acad. Sci. USA94: 5473–5477.
McCourt, P., Kunst, L., Browse, J. andSomerville, C.R. 1987. The effect of reduced amounts of lipid unsaturation on chloroplast ultrastructure and photosynthesis in a mutant ofArabidopsis. Plant Physiol84: 353–360.
Moons, A., Prinsen, E., Bauw, G. andVan Montagu, M. 1997. Antagonistic effects of abscisic acid and jasmonates on salt stress inducible transcripts in rice roots. Plant Cell9: 2243–2259.
Mueller, M.J., Brodschelm, W., Spannagl, E. andZenk, M.H. 1993. Signaling in the elicitation process is mediated through the octadecanoid pathway leading to jasmonic acid. Proc. Natl. Acad. Sci. USA90: 7490–7494.
Nishiuchi, T., Nishimura, M., Arondel, V. andIba, K. 1994. Genomic nucleotide sequence of a gene encoding a microsomal ω-3 fatty acid desaturase fromArabidopsis thaliana. Plant Physiol.105: 767–768.
Nishiuchi, T., Nakamura, T., Abe, T., Kodama, H., Nishimura, M. andIba, K. 1995. Tissue-specific and light-responsive regulation of the promoter region of theArabidopsis thaliana chloroplast ω-3 fatty acid desaturase gene (FAD7). Plant Mol. Biol.29: 599–609.
Nishiuchi, T., Hamada, T., Kodama, H. andIba, K. (1997). Wounding changes the spatial expression pattern of the Arabidopsis plastid ω-3 fatty acid desaturase gene (FAD7) through different signal transduction pathways. Plant Cell9: 1701–1712.
Peña-Cortés, H., Albrecht, T., Prat, S., Weiler, E.W. andWillmitzer, L. 1993. Aspirin prevents wound-induced gene expression in tomato leaves by blocking jasmonic acid biosynthesis. Planta191: 123–128.
Rushton, P.J., Torres, J.T., Parniske, M., Wernert, P., Hahlbrock, K. andSomssich, I.E. 1996. Interaction of elicitor-induced DNA-binding proteins with elicitor response elements in the promoters of parsley PR1 genes. EMBO J.15: 5690–5700.
Somerville, C. andBrowse, J. 1991. Plant lipids: Metabolism, mutants, and membranes. Science252: 80–87.
Thompson, W.F. andWhite, M.J. 1991. Physiological and molecular studies of light-regulated nuclear genes in higher plants, Annu. Rev. Plant Physiol. Plant Mol. Biol.42: 423–466.
Todoroki, S., Hayashi, T., Nagata, T., Kanegae, H., Mori, M. andKikuchi, S. 1998. cDNA cloning of gamma-ray inducible genes encoding ω-3 fatty acid desaturase from potato tuber. Plant Cell Physiol.39: S136.
Vick, B.A. andZimmerman, D.C. 1984. Biosynthesis of jasmonic acid by several plant species. Plant Physiol.75: 458–461.
Weber, H., Vick, B.A. andFarmer, E.E. 1997. Dinor-oxophytodienoic acid: A new hexadecanoid signal in the jasmonate family. Proc. Natl. Acad. Sci. USA94: 10473–10478.
Yadav, N.S., Wierzbicki, A., Aegerter, M., Caster, C.S., Pérez-Grau, L., Kinney, A.J., Hitz, W.D., Booth, J.R., Jr., Schweiger, B., Stecca, K.L., Allen, S.M., Blackwell, M., Reiter, R.S., Carlson, T.J., Russell, S.H., Feldmann, K.A., Pierce, J. andBrowse, J. 1993. Cloning of higher plant ω-3 fatty acid desaturases. Plant Physiol.103: 467–476.
Yamamoto, K.T., Mori, H. andImaseki, H. 1992 Novel mRNA sequences induced by indole 3 acetic acid in sections of elongating hypocotyls of mung bean (Vigna radiata). Plant Cell Physiol.33: 13–20.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nishiuchi, T., Iba, K. Roles of plastid ε-3 fatty acid desaturases in defense response of higher plants. J. Plant Res. 111, 481–486 (1998). https://doi.org/10.1007/BF02507782
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02507782