Summary
Over the years of chromatographic evolution, the purpose of using internal standards for quantitative determinations in bioanalysis has shifted from covering (correcting for) random and systematic errors of the entire method (including sample preparation, chromatography and detection) to currently covering (correcting for) random and systematic errors of the detection, by MS-MS especially. However, the need for internal standards in current LC-MS-MS is also dependent on the degree of chromatographic separation and the degree of sample clean-up. This emphasizes the need for integrated method development, including selection of an internal standard. Available options for internal standard selection are (1) no internal standard, (2) a structurally related compound, (3) a structurally similar compound or (4) a stable isotope labelled compound. An overview of the purposes of internal standards in bioanalytical LC-MS-MS is given and this overview is supported by various illustrations using different types of internal standards.
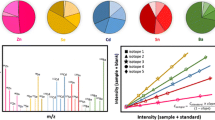
An overview of the purposes of internal standards in bioanalytical LC-MS-MS is given. It is supported by various illustrations using different types of internal standards.
The option of using deuterated internal standards has given us surprising results in some cases, where chromatographic and/or extraction behaviour of deuterated internal standards was different from the analyte.
Similar content being viewed by others
References
Curry, S.H.; Whelpton, R. inBlood Drugs and Other Analytical Challenges (Vol. 7, Methodological Surveys), Reid, E., Ed., Horwood/Wiley, Chichester,1978, 29–41.
Reid, E., as for [1], 61–76.
Vessman, J. inTrace-organic Sample Handling (Vol. 10, Methodological Surveys; now out-of-print), as for [1],1981, 341–347.
Dudley, K. H. as for [3], 336–340.
Wieling, J.; Dijkstra, H.; Mensink, C.K.; Jonkman, J.H.G.; Coenegracht P.M.J.; Doornbos, D.A.J. Chromatogr. 1993,629, 181–199.
Matuszewski, B.K.; Constanzer, M.L.; Chavez-Eng, C.M.Anal. Chem. 1998,70 (5) 882–889.
Lagerwerf, F.M.; van Dongen, W. D.; Steenvoorden, R.J.J.M.; Honing, M.; Jonkman, J.H.G.Trends in Analytical Chemistry 2000,19 (7), 418–427.
Hempenius, J.; Steenvoorden, R.J.J.M.; Lagerwerf, F.M.; Wieling, J.; Jonkman J.H.G.J. Pharm. Biomed. Anal. 1999,Sep. 20 (6), 889–98. cf. Wieling, J.; Hempenius, J.; Steenvoorden, R.J.J.M.; Jonkman, J.H.G.Proceedings of the 16th International Symposium on Laboratory Automation and Robotics October 18–21,1998.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wieling, J. LC-MS-MS experiences with internal standards. Chromatographia 55 (Suppl 1), S107–S113 (2002). https://doi.org/10.1007/BF02493365
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02493365