Summary
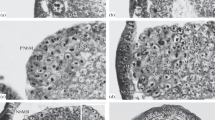
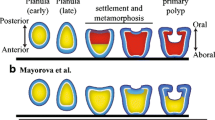
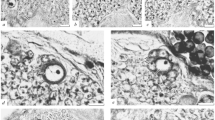
During embryogenesis and planula development of the colonial hydroidHydractinia echinata cell proliferation decreases in a distinct spatio-temporal pattern. Arrest in S-phase activity appears first in cells localized at the posterior and then subsequently at the anterior pole of the elongating embryo. These areas do not resume S-phase activity, even during the metamorphosis of the planula larva into the primary polyp. Tissue containing the quiescent cells gives rise to the terminal structures of the polyp. The posterior area of the larva becomes the hypostome and tentacles, while the anterior part of the larva develops into the basal plate and stolon tips. In mature planulae only a very few cells continue to proliferate. These cells are found in the middle part of the larva. Labelling experiments indicate that the prospective material of the postmetamorphic tentacles and stolon tips originates from cells which have exited from the cell cycle in embryogenesis or early in planula development. Precursor cells of the nematocytes which appear in the tentacles of the polyp following metamorphosis appear to have ceased cycling before the 38th hour of embryonic development. The vast majority of the cells that constitute the stolon tips of the primary polyp leave the cell cycle not later than 58 h after the beginning of development. We also report the identification of a cell type which differentiates in the polyp without passing through a post-metamorphic S-phase. The cell type appears to be neural in origin, based upon the identification of a neuropeptide of the FMRFamide type.
Similar content being viewed by others
References
Berking S (1984) Metamorphosis inHydractinia echinata. Insights into pattern formation in hydroids. Roux's Arch Dev Biol 193:370–378
Berking S (1986) Is homarine a morphogen in the marine hydroidHydractinia? Roux's Arch Dev Biol 195:33–38
Berking S (1987) Homarine (N-methylpicolinic acid) and trigonelline (N-methylnicotinic acid) appear to be involved in pattern control in a marine hydroid. Development 99:1–9
Berking S (1988) Ammonia, tetraethylammonium, barium and amiloride induce metamorphosis in the marine hydroidHydractinia. Roux's Arch Dev Biol 197:1–9
Eiben R (1982) Storage of embryonically transcribed poly(A) RNA and its utilization during metamorphosis of the hydroidHydractinia echinata. Roux's Arch Dev Biol 191:270–276
Fujisawa T, David CN (1982) Commitment during stenotele differentation inHydra is localized near the S/G2 boundary in terminal cell cycle. Dev Biol 93:226–230
Grimmelikhuijzen CJP (1985) Antisera to the sequence Arg-Pheamide visualise neuronal centralisations in hydroid polyps. Cell Tissue Res 241:171–181
Grimmelikhuijzen CJP, Graff D (1985) Arg-Phe-amide-like peptides in the primitive nervous system of coelenterates. Peptides 6:477–483
Koizumi O, Bode HR (1986) Plasticity in the nervous system of adultHydra I. The position-dependent expression of FMRFamide-like immunoreactivity. Dev Biol 116:407–421
Koizumi O, Heimfield S, Bode HR (1988) Plasticity in the nervous system of adultHydra II. Conversion of ganglion cells of the body column into epidermal sensory cells of the hypostome. Dev Biol 129:358–371
Lange R, Plickert G, Müller WA (1989) Histoincompatibility in a low invertebrate,Hydractinia echinata: Analysis of the mechanism of rejection. J Exp Zool 249:284–292
Lesh-Laurie G, Frank WN (1977) The influence of BrdU on interstitial cell differentation inHydra. Separatum Experentia 33:909
Müller WA (1984) Retinoids and pattern formation in a hydroid. J Embryol Exp Morphol 81:253–271
Müller WA, Spindler KD (1972) The effects of sulphydryl reagents on morphogenesis in hydroids I. Metamorphosis and regeneration inHydractinia echinata. Roux's Arch Dev Biol 170:152–164
Müller WA, Wieker F, Eiben R (1976) Larval adhesion, releasing stimuli and metamorphosis. In: Mackie GO (ed) Coelenterate ecology and behavior. Plenum Press, New York, pp 339–346
Müller WA, Mitze A, Wickhorst JP, Meier-Menge HM (1977) Polar morphogenesis in early hydroid development. Action of cesium, of neurotransmitters and of an intrinsic head activator on pattern formation. Roux's Arch Dev Biol 182:311–328
O'Sullivan N (1985) Untersuchungen zur Entwicklung des Nervensystems vonHydractinia echinata. Diploma Thesis, University of Heidelberg
Plickert G (1987) Low-molecular-weight factors from colonial hydroids affect pattern formation. Roux's Arch Dev Biol 196:248–256
Plickert G (1989) Proportion altering factor (PAF) stimulates nerve cell formation inHydractinia echinata. Cell Diff Dev 26:19–28
Plickert G, Kroiher M (1985) Analysis of cell proliferation kinetics by means of BrdU/anti-BrdU technique. Application to whole mounts ofHydractinia echinata. Abstr Eur J Cell Biol 39:27
Plickert G, Kroiher M (1988) Proliferation kinetics and cell lineages can be studied in whole mounts and macerated tissues by means of BrdU/anti-BrdU technique. Development 103:791–794
Plickert G, Kroiher M, Munck A (1988) Cell proliferation and early differentiation during embryonic development and metamorphosis ofHydractinia echinata. Development 103:795–803
Schaller HC (1976) Action of the head activator on the determination of interstitial cells inHydra. Cell Diff 5:13–20
Schwoerer-Böhning B, Kroiher M, Müller WA (1990) Signal transmission and covert prepattern in the metamorphosis ofHydractinia echinata (Hydrozoa). Roux's Arch Dev Biol 198:245–251
Spindler KD, Müller WA (1972) Induction of metamorphosis by bacteria and by a lithium pulse in the larvae ofHydractinia echinata (Hydrozoa). Roux's Arch Dev Biol 169:271–280
Van de Vyver G (1964) Etude histologique du développement d'Hydractinia echinata (Flem). Cah Biol Mar 5:295–310
Van de Vyver G (1967) Etude du développement embryonnaire des hydraires athécates (gymnoblastiques) à gonophores I. Formes à planula. Arch Biol (Liège) 78:451–518
Van de Vyver G (1980) A comparative study of the embryonic development in Hydrozoa athecata. In: Tardent P, Tardent R (ed) Developmental and cellular biology in coelenterates. Elsevier/North-Holland Biomedical Press, Amsterdam New York Oxford, pp 109–120
Venugopal G, David CN (1981) Nerve commitment inHydra II. Localization of commitment in S-Phase. Dev Biol 83:361–365
Weis VM, Douglas RK, Buss LW (1985) Biology of Hydractiniid hydroids 4. Ultrastructure of the planula ofHydractinia echinata. Biol Bull 168:403–418
Yaross MS, Maca BA, Chow MH, Bode HR (1982) Commitment ofHydra interstitial cells to nerve cell differentation occurs by late S-Phase. Dev Biol 89:425–436
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kroiher, M., Plickert, G. & Müller, W.A. Pattern of cell proliferation in embryogenesis and planula development ofHydractinia echinata predicts the postmetamorphic body pattern. Roux's Arch Dev Biol 199, 156–163 (1990). https://doi.org/10.1007/BF01681488
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01681488