Summary
-
1.
ERG S(λ) were determined in darkadapted intact preparations of 6 North American firefly species (Photinus collustrans, marginellus, pyralis, macdermotti, scintillans and Bicellonycha wickershamorum) which restrict their flashing activity to twilight hours. The curves possess narrow (1/2 bandwidth=50–60 nm) peaks in the yellow (560–580 nm) and a shoulder in the violet (370–420 nm), with amarked attenuation (1.4–2.2 log units) of sensitivity in the green (480–530 nm) region of the spectrum (Fig. 1). Two additional species (Photuris potomaca and frontalis) which initiate flashing at twilight and continue on late into the night (twi-night) possess broad sensitivity maxima around 560 nm (Fig. 3).
-
2.
Selective adaptation experiments isolated near-UV and yellow inP. scintillans (Fig. 2). In the dorsal frontal region of the compound eyes inP. frontalis, high sensitivity existed only in the short wavelength region (near-UV and blue) with a maximum in the blue (λ max 435 nm) (Fig. 4).
-
3.
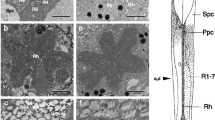
The in situ MSP absorption spectrum of the screening pigments was determined in preparations of firefly retina. a) Two kinds of dark brown granules were found in the clear zone region. These granules absorb all across the spectrum with a gradual increase in optical density in the shorter wavelength region inP. pyralis (Fig. 5). b) Besides dark granules, pink-to-red colored screening pigments were present in the vicinity of the rhabdoms. The absorption spectra of these pigments determined in five species were narrow (1/2 bandwidth=50–80 nm) with species-specific differences in their peak absorption in the green at 525 nm, 510 nm, 512 nm and 517 nm inP. scintillans, macdermotti, collustrans and pyralis, respectively (Fig. 6). A similar pigment was found inP. marginellus with aλ max at 512 nm (Fig. 7). In all cases, transmission increased both at long and short wavelengths, but more sharply in the long wavelength region (Figs. 6 and 7). Hence each twilight-restricted species has its own unique colored screening pigment. A yellow pigment whose absorption spectrum differed from those found in genusPhotinus was found in twi-night activePhoturis potomaca (λ max 461 nm) and night-activeP. versicolor (λ max 456 nm). The transmission of thePhoturis pigment increased sharply only in the long wavelength region (Fig. 8).
-
4.
In the twilight-restricted species, pink-to-red screening pigments modify dramatically the long green wavelength part of S(λ) functions. The calculated effect of the absorption of these screening pigments (O.D.=1.6 to 2.2; ¯X=1.8, n=4) on a theoretical S(λ) curve represented by a green (P550) rhodopsin, match the shape of the experimentally obtained dark-adapted ERG S(λ) in all cases (Figs. 9, 10). These screening pigments (Figs. 6, 7, 8) then would cause attenuation of sensitivity selectively in the green in twilight-restricted fireflies (Fig. 1) with a concomitant shifting of the peak of the sensitivity in the yellow as well as a narrowing of the visual spectral sensitivity. The pink-to-red colored screening pigments presumably would enhance color and/or brightness contrast in the mesopic range of ambient illumination.
-
5.
The presence of the colored screens attenuates absolute sensitivity from 5–25% among different twilight-active species as compared to a night-activePhoturis lucicrescens (Fig. 11).
Similar content being viewed by others
Abbreviations
- MSP :
-
microphotospectrometer
References
Aepli G, Labhart T, Meyer EP (1985) Structural specializations of the cornea and retina at the dorsal rim of the compound eye in hymenopteran insects. Cell Tissue Res 239:19–24
Bowmaker JK (1979) Visual pigments and oil droplets in the pigeon retina, as measured by microspectrophotometry and their relationship to spectral sensitivity. In: Granda AM, Maxwell JH (eds) Neural mechanisms of behavior in the pigeon. Plenum, New York, pp 287–305
Casella AJ, Strother GK, Connolly JW (1975) Simplified recording microspectrophotometer. Appl Opt 14:771–777
Cronin TW (1985) The visual pigment of a stomatopod crustacean,Squilla empusa. J Comp Physiol A 156:679–687
Ebrey TG, Honig B (1977) New wavelength dependent visual pigment nomograms. Vision Res 17:147–151
Eguchi E, Nemoto A, Meyer-Rochow VB, Ohba N (1984) A comparative study of spectral sensitivity curves in three diurnal and eight nocturnal species of Japanese fireflies. J Insect Physiol 30:607–612
Goldsmith TH (1978) The effects of screening pigments on the spectral sensitivity of some Crustacea with scotopic (superposition) eyes. Vision Res 18:475–482
Goldsmith TH, Collins JS, Licht S (1984) The cone oil droplets of avian retinas. Vision Res 24:1661–1671
Goldstein EB, Williams TP (1966) Calculated effects of ‘screening pigments’. Vision Res 6:39–50
Granda AM, Haden KW (1970) Retinal oil globule counts and distributions in two species of turtles:Pseudemys scripta elegans (Wied) andChelonia mydas mydas (L.). Vision Res 10:79–84
Höglund G (1966) Pigment migration, light screening and receptor sensitivity in the compound eye of nocturnal Lepidoptera. Acta Physiol Scand 69 [Suppl 282]: 1–56
Höglund G, Langer H, Struwe G, Thorell B (1970) Spectral absorption by screening pigment granules in the compound eyes of a moth and a wasp. Z Vergl Physiol 67:238–242
Höglund G, Hamdorf K, Rosner G (1973) Trichromatic visual system in an insect and its sensitivity control by blue light. J Comp Physiol 86:265–279
Labhart T (1980) Specialized photoreceptors at the dorsal rim of the honeybee's compound eye: Polarizational and angular sensitivity. J Comp Physiol 141:19–30
Labhart T, Hodel B, Valenzuela I (1984) The physiology of the cricket's compound eye with particular reference to the anatomically specialized dorsal rim area. J Comp Physiol A 155:289–296
Lall AB (1981a) Electroretinogram and the spectral sensitivity of the compound eyes in the fireflyPhoturis versicolor (Coleoptera-Lampyridae): A correspondence between green sensitivity and species bioluminescence emission. J Insect Physiol 27:461–468
Lall AB (1981b) Vision tuned to species bioluminescence emission in fireflyPhotinus pyralis. J Exp Zool 216:317–319
Lall AB, Chapman RM, Trouth CO, Holloway JA (1980a) Spectral mechanisms of the compound eye in the fireflyPhotinus pyralis (Coleoptera; Lampyridae). J Comp Physiol 135:21–27
Lall AB, Seliger HH, Biggley WH, Lloyd JE (1980b) Ecology of colors of firefly bioluminescence. Science 210:560–562
Lall AB, Lord ET, Trouth CO (1982) Vision in the fireflyPhoturis lucicrescens (Coleoptera: Lampyridae): Spectral sensitivity and selective adaptation in the compound eye. J Comp Physiol 147:195–200
Lall AB, Strother GK, Ribi W, Seliger HH, Chapados P, Lloyd JE (1984) In situ MSP measurements of screening pigments in rhabdomeric slices and their effect on the visual spectral sensitivity in twilight active fireflies. Abstract ARVO 1984. Invest Ophthalmol Visual Sci [Suppl] 25:219
Lall AB, Lord ET, Trouth CO (1985) Electrophysiology of the visual system in the cricketGryllus firmus (Orthoptera: Gryllidae): Spectral sensitivity of the compound eyes. J Insect Physiol 31:353–357
Lall AB, Seliger HH, Strother GK, Cronin T, Lloyd JE (1985) Modification of visual spectral sensitivity by colored screening pigments in the compound eyes of some twilight active fireflies. Abstract ARVO 1985. Invest Opththalmol Visual Sci [Suppl] 26:114
Land MF (1981) Optics and vision in invertebrates. In: Autrum H (ed) Vision in invertebrates (Handbook of sensory physiology, vol VIIB). Springer, Berlin Heidelberg New York, pp 471–593
Langer H (1975) Properties and functions of screening pigments in insect eyes. In: Snyder AW, Menzel R (eds) Photoreceptor optics. Springer, Berlin Heidelberg New York, pp 429–455
Lipetz LE (1984) Pigment types, densities and concentrations in cone oil droplets ofEmydoidea blandingii. Vision Res 24:605–612
Lythgoe JN (1968) Visual pigments and visual range underwater. Vision Res 8:997–1012
Lythgoe JN (1984) Visual pigments and environmental light. Vision Res 24:1539–1550
Meinecke CC (1981) The fine structure of the compound eye of the African armyworm moth,Spodoptera exempta Walk. (Lepidoptera, Noctuidae). Cell Tissue Res 216:333–347
Ribi WA (1978) Colour receptors in the eye of the digger wasp,Sphex cognatus Smith: evaluation by selective adaptation. Cell Tissue Res 195:471–483
Ribi WA (1979) Coloured screening pigments cause red eye glow in pierid butterflies. J Comp Physiol 132:1–9
Seliger HH, Lall AB, Lloyd JE, Biggley WH (1982a) On the colors of firefly bioluminescence. I. An optimization model. Photochem Photobiol 36:673–680
Seliger HH, Lall AB, Lloyd JE, Biggley WH (1982b) On the colors of firefly bioluminescence. II. Experimental evidence for the optimization model. Photochem Photobiol 36:681–688
Sillman AJ, Bolnick DA, Haynes LW, Walter AE, Loew ER (1981) Microspectrophotometry of the photoreceptors of palaeognathous birds, the Emu and the Tinamou. J Comp Physiol 144:271–276
Stowe S (1980) Spectral sensitivity and retinal pigment movement in the crabLeptograpsus variegatus (Fabricius). J Exp Biol 87:73–98
Strother GK, Casella AJ (1972) Microspectrophotometry of arthropod visual screening pigments. J Gen Physiol 59:616–636
Strother GK, Superdock DA (1972) In situ absorption spectra ofDrosophila melanogaster visual screening pigments. Vision Res 12:1545–1547
Wunderer H, Smola U (1982) Fine structure of ommatidia at the dorsal eye margin ofCalliphora erythrocephala Meigen (Diptera: Calliphoridae): An eye region specialized for the detection of polarized light. Int J Insect Morphol Embryol 11:25–38
Author information
Authors and Affiliations
Additional information
Contribution No. 1357 of the McCollum-Pratt Institue and Department of Biology, The Johns Hopkins University
Rights and permissions
About this article
Cite this article
Lall, A.B., Strother, G.K., Cronin, T.W. et al. Modification of spectral sensitivities by screening pigments in the compound eyes of twilight-active fireflies (Coleoptera: Lampyridae). J. Comp. Physiol. 162, 23–33 (1988). https://doi.org/10.1007/BF01342700
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01342700