Abstract
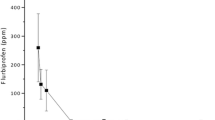
Flurbiprofen, a nonsteroidal antiinflammatory agent which is not ocularly metabolized, was employed as a probe compound to investigate the drug kinetic relationship between systemic and ocular humoral circulation. The ocular and systemic bioavailabilities of topically applied flurbiprofen were also quantitated. Anesthetized albino female rabbits received flurbiprofen doses intracamerally, topically, and intravenously at 2 to 4 week intervals. Aqueous humor and plasma were used as the sampling compartments. Plasma clearance values of flurbiprofen were 6.77 and 7.87 ml/min, after 6-mg and 208-μg intravenous doses, respectively. These values were not significantly different and indicated no dose-dependent disposition kinetics over a 30-fold dose range. Both ocular and systemic flurbiprofen dispositions followed a biexponential pattern with a rapid distribution phase. The systemic and ocular distribution half-lives of flurbiprofen were 12 min and 15 min, respectively. The plasma elimination half-life was 74 min and the aqueous humor elimination half-life was 93 min. The latter approximated the turnover rate of aqueous humor and suggested that aqueous humor drainage was the major process of flurbiprofen elimination from the globe. About 99% of flurbiprofen is bound to plasma protein. At distribution equilibrium, the plasma and aqueous humor concentrations of fluobiprofen differed by a hundredfold, suggesting that only free drug entered the aqueous humor after the administration of a systemic dose. In the ophthalmic studies, right eyes were instilled with 50 μl of 0.3% flurbiprofen in saline (dose = 150 μg), and left eyes were instilled with 50 μl of 0.15% flurbiprofen in saline (dose=75 μg). When the area of the aqueous humor concentration-versus-time curve values was normalized by the administered dose, the 75-μg dose was 30% more available to ocular tissues than was the 150-μg dose. This demonstrated a disproportionate relationship between the administered dose and the fraction absorbed. The intracameral dose was considered to be completely bioavailable for intraocular effects. The ocular bioavailability of the ophthalmic dose was defined by using intracameral administration as a standard measurement. The ocular bioavailabilities of the 75-μg and 150-μg topical flurbiprofen doses were 10% and 7%, respectively. Systemic bioavailability after topical administration of 225 μg of flurbiprofen was 74%.
Similar content being viewed by others
References
V. F. Smolen and R. D. Schoenwald. Drug-absorption analysis from pharmacological data. I: Method and confirmation exemplified for the mydriatic drug tropicamide.J. Pharm. Sci. 60:96–103 (1971).
T. J. Mikkelson, S. S. Chrai, and J. R. Robinson. Altered bioavailability of drugs in the eye due to drug-protein interaction.J. Pharm. Sci. 62:1648–1653 (1973).
V. F. Smolen, J. M. Clevender, E. J. Williams, and M. W. Bergdolt. Biophasic availability of ophthalmic carbachol I: Mechanism of cationic polymer and surfactant-promoted miotic activity.J. Pharm. Sci. 62:958–961 (1973).
M. Lee and E. R. Hammarlund. Corneal absorption of ophthalmic drugs.J. Pharm. Sci. 63:721–724 (1974).
H. Benson. Permeability of the cornea to topically applied drugs.Ophthamology 91:313–327 (1974).
S. S. Chrai and J. R. Robinson. Corneal permeation of topical pilocarpine nitrate in the rabbit.Am. J. Ophthalmol. 77:735–739 (1974).
T. F. Patton and M. Francoeur, Ocular bioavailability and systemic loss of topically applied ophthalmic drugs.Am. J. Ophthalmol. 85:225–229 (1978).
K. J. Himmelstein, I. Guvenir, and T. F. Patton. Preliminary pharmacokinetic model of pilocarpine uptake and distribution in the eye.J. Pharm. Sci. 67:603–608 (1978).
J. W. Shell. Pharmacokinetics of topically applied ophthalmic drugs.Surv. Ophthalmol. 26:207–218 (1982).
H. M. Leibowitz and A. Kupferman. Bioavailability and therapeutic effectiveness of topically administered corticosteroids.Trans. Am. Acad. Ophth. Otol. 79:78–88 (1975).
J. W. Sieg and J. R. Robinson. Vehicle effects on ocular drug bioavailability I: Evaluation of fluorometholone.J. Pharm. Sci. 64:931–936 (1975).
J. W. Sieg and J. R. Robinson. Vehicle effects on ocular drug bioavailability II: Evaluation of pilocarpine.J. Pharm. Sci. 66:1222–1228 (1977).
H. M. Leibowitz, A. R. Berrospi, A. Kupferman, G. V. Restropo, V. Galvis, and J. A. Alvarez. Penetration of topically administered prednisolone acetate into the human aqueous humor.Am. J. Ophthalmol. 83:402–406 (1977).
M. Gibaldi and D. Perrier.Pharmacokinetics, Vol. I. Marcel Dekker, New York, 1975.
J. A. Anderson, C. C. Chen, J. B. Vita, and M. Shackleton. Disposition of topical flurbiprofen in normal and aphakic rabbit eyes.Arch. Ophthalmol. 100:642–645 (1982).
L. Z. Benet and R. L. Galeazzi. Noncompartmental determination of the steady-state volume of distribution.J. Pharm. Sci. 68:1071–1074 (1979).
R. A. Moses (ed.).Adler's Physiology of the Eye, 7th ed. C. V. Mosby, St. Louis, 1981.
J. M. Conrad and J. R. Robinson. Aqueous chamber drug distribution volume measurement in rabbits.J. Pharm. Sci. 66:219–224 (1977).
S. M. Podos and B. Becker. Comparison of ocular prostaglandin synthesis inhibitors.Invest. Ophthalmol. 15:841–844 (1976).
S. M. Podos. Prostaglandins, nonsteroidal anti-inflammatory agents and eye disease.Trans. Am. Ophthal. Soc. 74:637–660 (1977).
S. L. Beal and L. B. Sheiner.NONMEN Users Guide-Part I, Users Basic Guide. Division of Clinical pharmacology, University of California, San Francisco, 1979.
L. B. Sheiner and S. L. Beal. Evaluation of methods for estimating population pharmacokinetic parameters. I. Michaelis-Menten Model: routine clinical pharmacokinetic data.J. Pharmacokin. Biopharm. 8:553–571 (1980).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tang-Liu, D.D.S., Liu, S.S. & Weinkam, R.J. Ocular and systemic bioavailability of ophthalmic flurbiprofen. Journal of Pharmacokinetics and Biopharmaceutics 12, 611–626 (1984). https://doi.org/10.1007/BF01059556
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF01059556