Summary
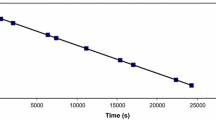
Rate constants are reported for reaction of the 4-cyanopyridine complexes [Fe(CN)5(4CNpy)]3− and [Mo(CO)5(4CNpy)] with a variety of incoming ligands, in aqueous methanol (40 vol % MeOH) and in toluene respectively, at 298.2 K (ambient pressure). The dependence of rate constants on the nature and concentration of the incoming ligand is discussed in terms of the operation of the limiting dissociative,D, mechanism for substitution; the operation of this mechanism here, and in analogous pentacyanoferrate(II), pentacarbonylmolybdenum(I), and penta- and tetra-cyanocobaltate(III) complexes is reviewed. The effect of pressure on rate constants for replacement of 4-cyanopyridine in [Mo(CO)5(4CNpy)], in toluene solution at 298.2 K, indicates an activation volume of +3 cm3 mol−1.
Similar content being viewed by others
References
C. H. Langford and H. B. Gray,Ligand Substitution Processes, Benjamin, New York, 1965.
M. L. Tobe,Inorganic Reaction Mechanisms, Nelson, London, 1972, ch. 7; R. G. Wilkins,The Study of Kinetics and Mechanism of Reactions of Transition Metal Complexes, Allyn and Bacon, Boston, 1974, ch. 4.
A. Haim, R. J. Grassi, and W. K. Wilmarth,Adv. Chem. Ser.,49, 31 (1965).
P. H. Tewari, R. W. Gaver, H. K. Wilcox, and W. K. Wilmarth,Inorg. Chem.,6, 611 (1967).
J. Halpern and D. N. Hague,Inorg. Chem.,6, 2059 (1967).
S. C. F. Au-Yeung and D. R. Eaton,Inorg. Chem.,23, 1517 (1984).
B. F. G. Johnson, J. Lewis and M. V. Twigg,J. Chem. Soc., Dalton Trans., 241 (1974).
H.-T. Macholdt and H. Elias,Inorg. Chem.,23, 4315 (1984); H.-T. Macholdt, Ph.D. Thesis, Technische Hochschule Darmstadt, 1984.
A. J. Poë and M. V. Twigg,J. Chem. Soc., Dalton Trans., 1860 (1974).
L. I. B. Haines and A. J. Poë,J. Chem. Soc. (A), 2826 (1969).
M. Basato,J. Chem. Soc., Dalton Trans., 91 (1985).
C. M. Carr, D. M. Davies, M. Gower, L. A. P. Kane-Maguire, and D. A. Sweigart,J. Chem. Soc., Dalton Trans., 923 (1981).
E. B. Fleischer, S. Jacobs, and L. Mestichelli,J. Am. Chem. Soc.,90, 2527 (1968).
Z. Dokuzović, D. Pavlović, S. Ašperger, and I. Murati,J. Chem. Soc., Chem. Commun., 1060 (1984).
E. Antonini and M. Brunori,Haemoglobin and Myoglobin in their Reactions with Ligands, North-Holland Publishing, Amsterdam, 1971.
M. G. Burnett and W. M. Gilfillan,J. Chem. Soc., Dalton Trans., 1578 (1981).
A. Haim,Inorg. Chem.,21, 2887 (1982).
M. H. M. Abou-El-Wafa and M. G. Burnett,J. Chem. Soc., Chem. Commun., 833 (1983).
K. F. Miller and A. D. Wentworth,Inorg. Chem.,15, 1467 (1976).
J. Burgess,Mech. Inorg. Organomet. React.,1, 129 (1983);2, 190 (1984);3, 216 (1985);4, 239 (1986).
M. H. M. Abou-El-Wafa, M. G. Burnett, and J. F. McCullagh,J. Chem. Soc., Dalton Trans., 2083 (1986).
M. G. Burnett,Chem. Soc. Rev.,12, 267 (1983).
W. K. Wilmarth, J. E. Byrd, H. N. Po, H. K. Wilcox, and P. H. Tewari,Coord. Chem. Rev.,51, 181 (1983); W. K. Wilmarth, J. E. Byrd, and H. N. Po,Coord. Chem. Rev.,51, 209 (1983).
M. H. M. Abou-El-Wafa and M. G. Burnett,Polyhedron,3, 895 (1984).
H. E. Toma and J. M. Malin,Inorg. Chem.,12, 1039 (1973).
D. Pavlović, I. Murati, and S. Ašperger,J. Chem. Soc., Dalton Trans., 602 (1973).
H. E. Toma and J. M. Malin,Inorg. Chem.,13, 1772 (1974).
Z. Bradić, D. Pavlović, I. Murati, and S. Ašperger,J. Chem. Soc., Dalton Trans., 344 (1974).
Z. Bradić, M. Pribanić, and S. Ašperger,J. Chem. Soc., Dalton Trans., 353 (1975).
H. E. Toma,J. Inorg. Nucl. Chem.,37, 785 (1975).
M. A. Blesa, J. A. Olabe, and P. J. Aymonino,J. Chem. Soc., Dalton Trans., 1196 (1976).
H. E. Toma, J. M. Malin, and E. Giesbrecht,J. Chem. Soc., Dalton Trans., 1610 (1978).
N. V. Hrepic and J. M. Malin,Inorg. Chem.,18, 409 (1979).
D. H. Macartney and A. McAuley,J. Chem. Soc., Dalton Trans., 1780 (1981).
M. J. Blandamer, J. Burgess, and R. I. Haines,J. Chem. Soc., Dalton Trans., 244 (1978).
T. R. Sullivan, D. R. Stranks, J. Burgess, and R. I. Haines,J. Chem. Soc., Dalton Trans., 1460 (1977).
M. J. Blandamer, J. Burgess, K. W. Morcom, and R. Sherry,Transition Met. Chem.,8, 354 (1983).
H.-T. Macholdt and R. van Eldik,Transition Met. Chem.,10, 323 (1985).
A. L. Coelho, H. E. Toma, and J. M. Malin,Inorg. Chem.,22, 2703 (1985).
J. M. Malin, H. E. Toma, and E. Giesbrecht,J. Chem. Educ.,54, 385 (1977).
J. M. A. Hoddenbagh and D. H. Macartney,Inorg. Chem.,25, 2099 (1986).
M. J. Blandamer, J. Burgess and R. I. Haines,J. Chem. Soc., Dalton Trans., 1293 (1976); M.-L. Moran, F. Sanchez, and J. Burgess, unpublished work; J. Salas, M. Katz, and N. E. Katz,J. Sol. Chem.,12, 115 (1983).
G. C. Pedrosa, J. A. Salas, M. Katz, and N. E. Katz,J. Coord. Chem.,12, 145 (1983).
G. C. Pedrosa, N. L. Hernandez, N. E. Katz, and M. Katz,J. Chem. Soc., Dalton Trans., 2297 (1980).
D. H. Macartney and A. McAuley,Inorg. Chem.,18, 2891 (1979).
M. J. Schadt and A. J. Lees,Inorg. Chem.,25, 672 (1986).
L. H. Staal, D. J. Stufkens, and A. Oskam,Inorg. Chim. Acta,26, 255 (1978).
J. Burgess, J. G. Chambers, and R. I. Haines,Transition Met. Chem.,6, 145 (1981); R. bin Ali, J. Burgess, M. Kotowski, and R. van Eldik,Transition Met. Chem., accepted for publication (TMC 1645).
W. D. Cowey and T. L. Brown,Inorg. Chem.,12, 2820 (1973).
G. Schmidt, Diplomarbeit, Technische Hochschule Darmstadt, 1985.
R. Romeo, personal communication.
M. H. M. Abou-El-Wafa and M. G. Burnett,Inorg. Chim. Acta,86, L7 (1984).
R. Romeo, D. Minniti, and S. Lanza,Inorg. Chem.,19, 3663 (1980); and refs therein.
J. A. S. Howell, personal communication.
J. Burgess and A. E. Smith,Transition Met. Chem.,12, 140 (1987).
H. Daamen, H. van der Poel, D. J. Stufkens, and A. Oskam,Thermochim. Acta,34, 69 (1979); K. E. Lewis, D. M. Golden, and G. P. Smith,J. Am. Chem. Soc.,106, 3905 (1984).
D. J. Kenney, T. P. Flynn, and J. B. Gallini,J. Inorg. Nucl. Chem.,20, 75 (1961).
A. J. Lees and A. W. Adamson,J. Am. Chem. Soc.,104, 3804 (1982); S. Chun, E. E. Getty, and A. J. Lees,Inorg. Chem.,23, 2155 (1984).
H. V. Pechmann,Chem. Ber.,21, 1411 (1888); H. A. Sinn.Dissertation, Technische Hochschule Darmstadt, 1966.
J. Burgess and C. D. Hubbard,J. Am. Chem. Soc.,106, 1717 (1984); N. Hallinan, P. McArdle, J. Burgess, and P. Guardado,J. Organometal. Chem., accepted for publication.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Abu-Gharib, EE.A., Ali, R.b., Blandamer, M.J. et al. The limiting dissociative mechanism for substitution at thed 6 centres molybdenum(O), iron(II), and cobalt(III): Kinetics of substitution at the pentacyano-4-Cyanopyridineferrate(II) anion and at 4-cyanopyridinemolybdenum(I) pentacarbonyl. Transition Met Chem 12, 371–378 (1987). https://doi.org/10.1007/BF01024038
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01024038