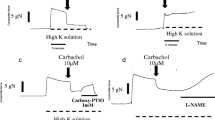
Abstract
• Background: This study addresses whether oxygen modulates the relaxation induced in retinal pericytes by sodium nitroprusside (SNP), a nitric oxide (NO) donor that stimulates the NO/guanylate cyclase pathway. • Methods:Bovine retinal pericytes were cultured on silicone. On the silicone surface, basal pericyte contractile tone induces wrinkles. Drug-induced changes in pericyte contractile tone were assessed by changes in the number of wrinkles. The effects of 100% nitrogen (hypoxia) and 100% oxygen (hyperoxia) were studied on: (a) the basal tone of quiescent pericytes, (b) the relaxation to 3 and 10 μM SNP or 1 μM forskolin, and (c) the recontraction that followed the washout of 3 μM SNP or 1 μM forskolin. • Results: Neither hypoxia nor hyperoxia had any apparent influence on pericyte basal tone, on forskolin-induced relaxation, or on pericyte recontraction after a forskolin-induced relaxation. In hypoxia, relaxations to SNP 3 μM (P<0.05) and 10 μM (P<0.01) were significantly more pronounced than in hyperoxia. Hypoxia also reduced the recontraction after an SNP-induced relaxation (P<0.001). • Conclusion: Oxygen modulates the relaxation of bovine retinal pericytes evoked by SNP (guanylate cyclase-mediated), but not the relaxation induced by forskolin (adenylate cyclase-mediated). These results suggest that in the retinal capillary circulation an interaction between oxygen and the NO/guanylate cyclase pathway modulates pericyte tone, and thus potentially blood flow.
Similar content being viewed by others
References
Aotaki-Keen AE, Harvey AK, de Juan E, Hjelmand LM (1991) Primary culture of human retinal glia. Invest Ophthalmol Vis Sci 32: 1733–1738
Anderson DR (1970) Vascular supply to the optic nerve of primates. Am J Ophthalmol 60: 341–351
Anderson DR (1970) Ultrastructure of the optic nerve head. Arch Ophthalmol 83: 63–73
Anderson DR (1996) Glaucoma, capillaries, and pericytes. 1. Blood flow regulation. Ophthalmologica 210: 257–262
Anderson DR, Braverman S (1976) Optic disc vasculature. Am J Ophthalmol 82: 165–174
Anderson DR, Davis EB (1996) Glaucoma, capillaries, and pericytes. 2. Identification and characterization of retinal pericytes in culture. Ophthalmologica 210: 263–268
D'Amore PA (1990) Culture and study of pericytes. In Piepr HM (ed) Cell culture techniques in cardiovascular research. Springer, Berlin Heidelberg New York, pp 299–314
Donati G, Pournaras CJ, Munoz JL, Poitry S, Poitry-Yamate CL, Tsacopoulos M (1995) Nitric oxide controls arteriolar tone in the retina of the miniature pig. Invest Ophthalmol Vis Sci 36: 2228–2237
Ferrari-Dileo G, Davis EB, Anderson DR (1992) Effects of cholinergic and adrenergic agonists on adenylate cyclase activity of retinal microvascular pericytes in culture. Invest Ophthalmol Vis Sci 33: 42–47
Frank RN, Dutta S, Mancini MA (1987) Pericyte coverage is greater in the retinal than in the cerebral capillaries of the rat. Invest Ophthalmol Vis Sci 28: 1086–1091
Frank RN, Turczyn TJ, Das A (1990) Pericyte coverage of retinal and cerebral capillaries. Invest Ophthalmol Vis Sci. 31: 999–1007
Haefliger IO, Anderson DR (1996) Blood-flow regulation in the optic nerve head. In: Ritch R, Shields MB, Krupin T (eds) The Glaucomas, 2nd edition. Mosby-Year Book, Inc., St Louis, MI, pp 189–197
Haefliger IO, Flammer J, Lüscher TF (1992) Nitric oxide and endothelin-1 are important regulators of the human ophthalmic artery. Invest Ophthalmol Vis Sci 33: 2340–2343
Haefliger IO, Meyer P, Flammer J, Löscher TF (1994) The vascular endothelium as a regulator of the ocular circulation: a new concept in ophthalmology? Surv Ophthalmol 39: 123–132
Haefliger IO, Zschauer A, Anderson DR (1994) Relaxation of retinal pericytes contractile tone through the nitric oxide cyclic guanosine monophosphate pathway. Invest Ophthalmol Vis Sci 35: 991–997
Harris AK, Wild P, Stopak D (1980) Silicone rubber substrata: A new wrinkle in the study of cell locomotion. Science 208: 177–179
Kelley C, D'Amore P, Hechtman HB, Shepro D (1987) Microvascular pericyte contractility in vitro: Comparison with other cells of the vascular wall. J Cell Biol 104: 483–490
Lipowsky HH, Kolvalcheck S, Zweifach BW (1978) The distribution of blood rheological parameters in the microvasculature of cat mesentery. Circ Res 43: 738–749
McLaren MJ, Inana G, Li CY (1993) Double fluorescent vital assay of phagocytosis by cultured retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 34: 317–326
Nayak RC, Berman AB, George KL, Eisenbarth GS, King GL (1988) A monoclonal antibody (3G5)-defined ganglioside antigen is expressed on the cell surface of microvascular pericytes. J Exp Med 167: 1003–1015
Rubanyi GM, Vanhoutte PM (1986) Superoxide anions and hyperoxia inactivate endothelium-derived relaxing factor. Am J Physiol 250: H822-H827
Tilton RG, Kilo C, Williamson JR, Murch DW (1979) Differences in pericyte contractile function in rat cardiac and skeletal muscle microvasculatures. Microvasc Res 18: 336–352
Voyata J, Via D, Butterfield C, Zetter B (1984) Identification and isolation of endothelial cells based on their increased uptake of acetylated-low density lipoproteins. J Cell Biol 99: 2034–2040
Wallow IH, Bindley CD, Reboussin DM, Gange SJ, Fisher MR (1993) Systemic hypertension produces pericyte changes in retinal capillaries. Invest Ophthalmol Vis Sci 34: 420–430
Wink DA, Beckman IS, Ford PC (1996) Kinetics of nitric oxide reaction in liquid and gas phase. In: Freelish M, Stamler S (eds) Methods in Nitric Oxide Research. John Wiley & Sons, Chichester, England, pp 29–37
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Haefliger, I.O., Chen, Q. & Anderson, D.R. Effect of oxygen on relaxation of retinal pericytes by sodium nitroprusside. Graefe's Arch Clin Exp Ophthalmol 235, 388–392 (1997). https://doi.org/10.1007/BF00937289
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00937289