Abstract
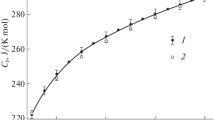
The heat capacity of glaucophane from the Sesia-Lanza region of Italy having the approximate composition (Na1.93Ca0.05Fe0.02) (Mg2.60Fe0.41) (Al1.83Fe0.15Cr0.01) (Si7.92Al0.08)O22(OH)2 was measured by adiabatic calorimetry between 4.6 and 359.4 K. After correcting the C 0p data to values for ideal glaucophane, Na2Mg3Al2Si8O22(OH)2 the third-law entropy S 0298 -S 00 was calculated to be 541.2±3.0 J·mol-1·K-1. Our value for S 0298 -S 00 is 12.0 J·mol-1·K-1 (2.2%) smaller than the value of Likhoydov et al. (1982), 553.2±3.0, is within 6.2 J·mol-1·K-1 of the value estimated by Holland (1988), and agrees remarkably well with the value calculated by Gillet et al. (1989) from spectroscopic data, 539 J·mol-1·K-1.
Similar content being viewed by others
References
Anderson CT (1937) The heat capacities of chromium, chromic oxide, chromous chloride, and chromic chloride at low temperatures. J Am Chem Soc 59:488–491
Bennington KO, Ferrante MJ, Stuve JM (1978) Thermodynamic data on the amphibole asbestos minerals amosite and crocidolite. US Bur Mines Rep Invest 8265
Carlin RL, van Duyneveldt AJ (1977) Magnetic properties of transition metal compounds. Springer, New York, Heidelberg, Berlin
Carman JH, Gilbert MC (1983) Experimental studies on glaucophane stability. Am J Sci 283A:414–437
Gillet Ph, Reynard B, Tequi C (1989) Thermodynamic properties of glaucophane, new data from calorimetric and spectroscopic measurements. Phys Chem Minerals 16:659–667
Gopal ESR (1966) Specific heats at low temperatures. Plenum, New York
Gronvold F, Westrum EF Jr (1959) α-ferric oxide: low temperature heat capacity and thermodynamic functions. J Amer Chem Soc 81:1780–1783
Hatton WE, Hildenbrand DL, Sinke GC, Stull DR (1959) The chemical thermodynamic properties of calcium hydroxide. J Am Chem Soc 81:5028–5030
Holland TJB (1988) Preliminary phase relations involving glaucophane and applications to high pressure petrology: new heat capacity and thermodynamic data. Contrib Mineral Petrol 99:134–142
Kelley KK, Todd SS, Orr RL, King EG, Bonnickson KR (1953) Thermodynamic properties of sodium-aluminum and potassium-aluminum silicates. US Bur Mines Rep Invest 4955
Ko HC, Ferrante MJ, Stuve JM (1977) Thermophysical properties of acmite. In: Cezairliyan A (ed) Proceedings of the Seventh Symposium on Thermophysical Properties. Am Soc Mech Engineers, New York, pp 392–395
Krupka KM, Robie RA, Hemingway BS, Kerrick DM, Ito J (1985) Low-temperature heat capacities and derived thermodynamic properties of anthophyllite, diopside, enstatite, bronzite and wollastonite. Am Mineral 70:249–260
Likhoydov GG, Sidorov YuI, Gurevich VM, Gorbunov VYe, Lennykh VI, Velizer PN, Khodakovskiy IL (1982) The thermodynamic parameters of glaucophane Na2Mg3Al2Si8O22(OH)2 and some petrological consequences. Geochem Int 19:66–77
Robie RA (1987) Calorimetry. In: Ulmer GC, Barnes HL (eds) Hydrothermal Experimental Techniques. Wiley-Interscience, New York, pp 389–422
Robie RA, Stout JW (1963) Heat capacity from 12 to 304° K and entropy of talc and tremolite. J Phys Chem 67:2252–2256
Robie RA, Hemingway BS (1984) Heat capacities and entropies of phlogopite (KMg3[AlSi3O10](OH)2) and paragonite (Na−Al2[AlSi3O10](OH)2) between 5 and 900 K and estimates of the enthalpies and Gibbs free energies of formation. Am Mineral 69:858–868
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Robie, R.A., Hemingway, B.S., Gillet, P. et al. On the entropy of glaucophane Na2Mg3Al2Si8O22(OH)2 . Contr. Mineral. and Petrol. 107, 484–486 (1991). https://doi.org/10.1007/BF00310682
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00310682