Summary
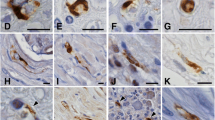
Patients with olivopontocerebellar atrophy (OPCA) were studied, and cytoplasmic inclusions were observed in some of the remaining neurons of the pontine nuclei, nuclei reticularis tegmenti pontis and arcuate nuclei. The cytoplasmic argyrophilic inclusions were demonstrated by silver impregnation techniques such as Bielschowsky and Bodian staining. With hematoxylin and eosin stain, the inclusions were sharply demarcated and appeared pale. The inclusions were not stained by the following routine histological methods: Klüver-Barrera, phosphotungstic acid hematoxylin, Holzer, periodic acid-Schiff, Mallory azan, alcian blue, nile blue, Masson trichrome, Congo red, thioflavine S, oil red O and Sudan black B stains. Immunohistochemistry with anti-ubiquitin antiserum showed that these inclusions were ubiquitinated. However, the inclusions did not react with any of the following antibodies (Abs) or antisera: anti-phosphorylated neurofilament (NF) Ab, anti-nonphosphorylated NF Abs (160 and 200 kDa), anti-paired helical filament antiserum, anti-tau antiserum, anti-tubulin Abs (alpha and beta), anti-microtubule-associated proteins antiserum, anti-glial fibrillary acidic protein antiserum, anti-vimentin Ab, anti-desmin Ab, anti-cytokeratin Abs (low and high molecular weights), anti-actin antiserum, anti-skeletal myosin antiserum and anti-myelin basic protein Ab. Ultrastructurally, the inclusion bodies noted in OPCA were composed primarily of fibrils having a width ranging from about 24 to 40 nm, which were entirely coated with osmiophilic granular material along their whole length. They were occasionally intermingled with a few filaments about 10 nm in width. Electron microscopical examination on silver-impregnated specimens revealed that each granule-coated fibril had a great affinity for silver particles. In elucidating the pathogenesis of OPCA, it was considered to be an important neuropathological finding that some of the remaining pontine neurons affected by OPCA developed characteristic cytoplasmic argyrophilic inclusions.
Similar content being viewed by others
References
Berciano J (1982) Olivopontocerebellar atrophy: a review of 117 cases. J Neurol Sci 53: 253–272
Carpenter MB, Sutin J, (1983) Human neuroanatomy, 8th edn. Williams & Wilkins, Baltimore, pp 315–409
Chokroverty S, Khedekar R, Derby B, Sachdeo R, Yook C, Lepore F, Nicklas W, Duvoisin RC (1984) Pathology of olivopontocerebellar atrophy with glutamate dehydrogenase deficiency. Neurology 34: 1451–1455
Ciechanover A, Finley D, Varshavsky A (1984) Ubiquitin dependence of selective protein degradation demonstrated in the mammalian cell cycle mutant ts85. Cell 37: 57–66
Déjérine J, Thomas A (1900) Latrophie olivo-ponto-cérébelleuse. Nouv Iconogr Salpet 13: 330–370
Duvoisin RC (1984) An apology and an introduction to the olivopontocerebellar atrophies. Adv Neurol 41: 5–12
Duvoisin RC, Chokroverty S, Lepore F, Nicklas W (1983) Glutamate dehydrogenase deficiency in patients with olivopontocerebellar atrophy. Neurology 33: 1322–1326
Essick CR (1912) The development of the nuclei pontis and the nucleus arcuatus in man. Am J Anat 13: 25–54
Finocchiaro G, Taroni F, Di Donato S (1986) Glutamate dehydrogenase in olivopontocerebellar atrophies: leukocytes, fibroblasts, and muscle mitochondria. Neurology 36: 550–553
Haugh MC, Probst A, Ulrich J, Kahn J, Anderton BH (1986) Alzheimer neurofibrillary tangles contain phosphorylated and hidden neurofilament epitopes. J Neurol Neurosurg Psychiatry 49: 1213–1220
Hayashi H (1985) Enzymatic analysis of individual posterior root ganglion cells in olivopontocerebellar atrophy, amyotrophic lateral sclerosis and Duchenne muscular dystrophy. J Neurol Sci 70: 13–20
Hershko A (1983) Ubiquitin: roles in protein modification and breakdown. Cell 34: 11–12
Hershko A, Eytan E, Ciechanover A, Haas AL (1982) Immunochemical analysis of the turnover of ubiquitin-protein conjugates in intact cells. J Biol Chem 257: 13964–13970
Ihara Y, Nukina N, Miura R, Ogawara M (1986) Phosphorylated tau protein is integrated into paired helical filaments in Alzheimer's disease. J Biochem 99: 1807–1810
Jellinger K, Tarnowska-Dziduszko E (1971) Die ZNS-Veränderungen bei den olivo-ponto-cerebellaren Atrophien. Z Neurol 199: 192–214
Koeppen AH, Barron KD (1984) The neuropathology of olivopontocerebellar atrophy. Adv Neurol 41: 13–38
Konagaya Y, Konagaya M, Takayanagi T (1986) Glutamate dehydrogenase and its isozyme activity in olivopontocerebellar atrophy. J Neurol Sci 74: 231–236
Kondo J, Honda T, Mori H, Hamada Y, Miura R, Ogawara M, Ihara Y (1988) The carboxyl third of tau is tightly bound to paired helical filaments. Neuron 1: 827–834
Kuzuhara S, Mori H, Izumiyama N, Yoshimura M, Ihara Y (1988) Lewy bodies are ubiquitinated: a light and electron microscopic immunocytochemical study. Acta Neuropathol (Berl) 75: 345–353
Lee S, Park YD, Yen SHC, Ksiezak-Reding H, Goldman JE, Dickson DW (1989) A study of infantile motor neuron disease with neurofilament and ubiquitin immunocytochemistry. Neuropediatrics 20: 107–111
Love S, Saitoh T, Quijada S, Cole GM, Terry RD (1988) Alz-50, ubiquitin and tau immunoreactivity of neurofibrillary tongles, Pick bodies and Lewy bodies. J Neuropathol Exp Neurol 47: 393–405
Lowe J, Blanchard A, Morrell K, Lennox G, Reynolds L, Billett M, Landon M, Mayer RJ (1988) Ubiquitin is a common factor in intermediate filament inclusion bodies of diverse type in man, inclusing those of Parkinson's disease, Pick's disease, and Alzheimer's disease, as well as Rosenthal fibers in cerebellar astrocytomas, cytoplasmic bodies in muscle, and Mallory bodies in alcoholic liver disease. J Pathol 155: 9–15
Menzel P (1891) Beitrag zur Kenntniss der hereditären Ataxie und Kleinhirnatrophie. Arch Psychiatr Nervenkr 22: 160–190
Mori H, Kondo J, Ihara Y (1987) Ubiquitin is a component of paired helical filaments in Alzheimer's disease. Science 235: 1641–1644
Munoz-Garcia D, Ludwin SK (1984) Classic and generalized variants of Pick's disease: a clinicopathological, ultrastructural, and immunocytochemical comparative study. Ann Neurol 16: 467–480
Nakazato Y, Sasaki A, Hirato J, Ishida Y (1987) Monoclonal antibodies which recognize phosphorylated and nonphosphorylated epitopes of neurofilament protein. Biomed Res 8: 369–376
Nukina N, Ihara Y (1986) One of the antigenic determinants of paired helical filaments is related to tau protein. J Biochem 99: 1541–1544
Oppenheimer DR (1984) Diseases of the basal ganglia, cerebellum and motor neurons. In: Adams JH, Corsellis JAN, Duchen LW (eds) Greenfield's neuropathology, 4th edn. Edward Arnold, London, pp 699–747
Pearse AGE (1972) Histochemistry, 3rd edn. Churchill, London, pp 1018–1023, p 1376
Perry G, Friedman R, Shaw G, Chau V (1987) Ubiquitin is detected in neurofibrillary tangles and senile plaque neurites of Alzheimer disease brains. Proc Natl Acad Sci USA 84: 3033–3036
Perry TL (1984) Four biochemically different types of dominantly inherited olivopontocerebellar atrophy. Adv Neurol 41: 205–216
Plaitakis A (1984) Abnormal metabolism of neuroexcitatory amino acids in olivopontocerebellar atrophy. Adv Neurol 41: 225–243
Sorbi S, Tonini S, Giannini E, Piacentini S, Martini P, Amaducci L (1986) Abnormal platelet glutamate dehydrogenase activity and activation in dominant and nondominant olivopontocerebellar atrophy. Ann Neurol 19: 239–245
Sternberger LA, Sternberger NH (1983) Monoclonal antibodies distinguish phosphorylated and nonphosphorylated forms of neurofilaments in situ. Proc Natl Acad Sci USA 80: 6126–6130
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kato, S., Nakamura, H. Cytoplasmic argyrophilic inclusions in neurons of pontine nuclei in patients with olivopontocerebellar atrophy: immunohistochemical and ultrastructural studies. Acta Neuropathol 79, 584–594 (1990). https://doi.org/10.1007/BF00294235
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00294235