Abstract
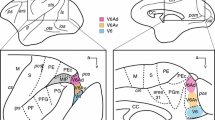
The striatopallidonigral connection was studied by injecting anterograde tracers into either the associative or the sensorimotor striatum in ten macaques. The results were analyzed using a precise cartographic method. Injections into various parts of the associative striatum (caudate nucleus and ventromedial putamen) produced a labeling of axons in the dorsomedial and ventral pallidal regions. These associative regions occupied two-thirds of the lateral pallidum and one-third of the medial pallidum. Bands of labeled axons from the sensorimotor striatum (dorsolateral putamen) were found in the remaining, central part of the two pallidal nuclei. In the substantia nigra, the rostral associative striatum projected medially to the pars reticulata, while the caudal parts projected laterally. The whole pars reticulata and lateralis thus appeared to receive associative striatal inputs. The sensorimotor striatal territory projected to the central part of the pars reticulata/lateralis. It was concluded that the two functional territories remain separate in the two pallidal nuclei but overlap in the middle third of the substantia nigra. However, due to their great size, the pallidal neurons located at the border of the two territories may receive striatal inputs from both the associative and the sensorimotor components in the same way that nigral neurons do.
Similar content being viewed by others
References
DeLong MR (1971) Activity of pallidal neurons during movement. J Neurophysiol 34:414–427
DeLong MR, Georgopoulos AP (1981) Motor functions of the basal ganglia. In: Brooks VB (ed) Motor control. (Handbook of physiology, sect 1. The nervous system, vol 2) American Physiological Society, Bethesda, pp 1017–1061
DeLong MR, Crutcher MD, Georgopoulos AP (1983) Relations between movement and single cell discharge in the substantia nigra of the behaving monkey. J Neurosci 3:1599–1606
DeLong MR, Crutcher MD, Georgopoulos AP (1985) Primate globus pallidus and subthalamic nucleus: functional organization. J Neurophysiol 53:530–543
Flaherty AW, Graybiel AM (1993) Two input systems for body representations in the primate striatal matrix: experimental evidence in the squirrel monkey. J Neurosci 13:1120–1137.
François C, Percheron G, Yelnik J, Heyner S (1985) A histological atlas of the macaque (Macaca mulatta) substantia nigra in ventricular coordinates. Brain Res Bull 14:349–367
François C, Yelnik J, Percheron G (1987) Golgi study of the primate substantia nigra. II. Spatial organization of dendritic arborizations in relation to the cytoarchitectonic boundaries and to the striatonigral bundle. J Comp Neurol 265:473–493
François C, Percheron G, Yelnik J (1992) Associative and sensorimotor parts of the pallidum and substantia nigra in macaques (abstract). Intern Basal Ganglia Soc, Giens, France, p 30
François C, Yelnik J, Percheron G, Tandé D (1994) Calbindin D-28k as a marker for the associative cortical territory of the striatum in macaques. Brain Res 633:331–336
Haber SN, Lynd E, Klein C, Groenewegen HJ (1990) Topographic organization of the ventral striatal efferent projections in the rhesus monkey: an anterograde tracing study. J Comp Neurol 293:282–298
Hamada I, DeLong MR, Mano N (1990) Activity of identified wrist-related pallidal neurons during step and ramp wrist movements in the monkey. J Neurophysiol 64:1892–1906
Hazrati L-N, Parent A (1992) The striatopallidal projection displays a high degree of anatomical specificity in the primate. Brain Res 592:213–227
Hedreen JC, DeLong MR (1991) Organization of striatopallidal, striatonigral, and nigrostriatal projections in the macaque. J Comp Neurol 304:569–595
Hikosaka O, Wurtz RH (1983) Visual and oculomotor functions of monkey substantia nigra pars reticulata. I. Relation of visual and auditory responses to saccades. J Neurophysiol 49:1230–1253
Hoesen GW van, Yeterian EH, Lavizzo-Mourney R (1981) Wide-spread corticostriate projections from temporal cortex of the rhesus monkey. J Comp Neurol 199:205–219
Jones EG, Coulter JD, Burton H, Porter R (1977) Cells of origin and terminal distribution of corticostriatal fibers arising in the sensory-motor cortex of monkeys. J Comp Neurol 173:53–80
Künzle H (1975) Bilateral projections from precentral motor cortex to the putamen and other parts of the basal ganglia. An autoradiographic study in Macaca fascicularis. Brain Res 88:195–209
Künzle H (1977) Projections from the primary somatosensory cortex to basal ganglia and thalamus in the monkey. Exp Brain Res 30:481–492
Künzle H (1978) An autoradiographic analysis of the efferent connections from premotor and adjacent prefrontal regions (areas 6 and 9) in Macaca fascicularis. Brain Behav Evol 15:185–234
Künzle H, Akert K (1977) Efferent connections of cortical, area 8 (frontal eye field) in Macaca fascicularis. A reinvestigation using the autoradiographic technique. J Comp Neurol 173:147–164
Lynd-Balta E, Mitchell SJ, Haber SN (1991) Organization of nigrostriatal and striatonigral projections in the primate. Soc Neurosci Abstr 17:1300
Mesulam MM (1978) Tetramethyl benzidine for horseradish peroxidase neurohistochemistry: a non-carcinogenic blue reaction-product with superior sensitivity for visualizing neural afferents and efferents. J Histochem Cytochem 26:106–117
Nauta WJH, Mehler WR (1966) Projections from the lentiform nucleus in the monkey. Brain Res 1:3–42
Ohye C, Le Guyader C, Féger J (1976) Responses of subthalamic and pallidal neurons to striatal stimulation: an extracellular study on awake monkeys. Brain Res 111:241–252
Parent A, Bouchard C, Smith Y (1984) The striatopallidal and striatonigral projections: two distinct fiber systems in primate. Brain Res 303:385–390
Parthasarathy HB, Schall JD, Graybiel AM (1992) Distributed but convergent ordering of corticostriatal projections: analysis of the frontal eye field and the supplementary eye field in the macaque monkey. J Neurosci 12:4468–4488
Percheron G, Yelnik J, François C (1984a) A Golgi analysis of the primate globus pallidus. III. Spatial organization of the striato-pallidal complex. J Comp Neurol 227:214–227
Percheron G, Yelnik J, François C (1984b) The primate striato-pallido-nigral system: an integrative system for cortical information. In: McKenzie JS, Kemm RE, Wilcock LN (eds) The basal ganglia. Plenum, New York, pp 87–105
Percheron G, François C, Yelnik J (1986) Instruments and techniques for the stereotactic surgery based on the CA-CP system of coordinates in monkeys. J Neurosci Methods 17:89–99
Percheron G, François C, Yelnik J, Fénelon G (1989) The primate nigro-striato-pallido-nigral system. Not a mere loop. In: Crossman A, Sambrook M (eds) Neural mechanisms in disorders of movement. Libbey, London, pp 103–109
Percheron G, Yelnik J, François C, Fénelon G, Talbi B (1991) The patial organisation of information processing in the striatopallido-nigral system. In: Bignami A (ed) Basal ganglia and movement disorders. (New Issues in neuroscience, vol III) Wiley, Chichester, pp 211–234
Rolls ET, Johnstone S (1992) Neurophysiological analysis of striatal function. In: Vallar G, Cappa SF, Wallesch CW (eds) Neuropsychological disorders associated with subcortical lesions. Oxford University Press, Oxford, pp 61–97
Rye DB, Saper CB, Wainer BH (1984) Stabilization of the tetramethylbenzidine (TMB) reaction product: application for retrograde and anterograde tracing, and combination with immunohistochemistry. J Histochem Cytochem 32:1145–1153
Saint-Cyr JA, Ungerleider LG, Desimone R (1990) Organization of visual cortical inputs to the striatum and subsequent outputs to the pallido-nigral complex in the monkey. J Comp Neurol 298:129–156
Schultz W (1986) Activity of pars reticulata neurons of monkey substantia nigra in relation to motor, sensory, and complex events. J Neurophysiol 55:660–677
Selemon LD, Goldman-Rakic PS (1988) Common cortical and subcortical targets of the dorsolateral prefrontal and posterior parietal cortices in the rhesus monkey: evidence for a distributed neural network subserving spatially guided behavior. J Neurosci 8:4049–4068
Selemon LD, Goldman-Rakic PS (1990) Topographic intermingling of striatonigral and striatopallidal neurons in the rhesus monkey. J Comp Neurol 297:359–376
Smith Y, Parent A (1986) Differential connections of caudate nucleus and putamen in the squirrel monkey (Säimiri sciureus). Neuroscience 18:347–371
Szabo J (1962) Topical distribution of the striatal efferents in the monkey. Exp Neurol 5:21–36
Szabo J (1967) The efferent projections of the putamen in the monkey. Exp Neurol 19:463–476
Szabo J (1970) Projections from the body of the caudate nucleus in the rhesus monkey. Exp Neurol 27:1–15
Tremblay L, Filion M (1989) Responses of pallidal neurons to striatal stimulations in intact waking monkeys. Brain Res 498:1–16
Wiesendanger M, Wiesendanger R (1984) The supplementary motor area in the light of recent investigations. Exp Brain Res [Suppl] 9:382–392
Yelnik J, Percheron G, François C (1984) A Golgi analysis of the primate globus pallidus. II. Quantitative morphology and spatial organization of dendritic arborizations. J Comp Neurol 227:200–213
Yelnik J, François C, Percheron G, Heyner S (1987) Golgi study of the primate substantia nigra. I. Quantitative morphology and typology of nigral neurons. J Comp Neurol 265:455–472
Yeterian EH, Hoesen GW van (1978) Cortico-striate projections in the rhesus monkey: the organization of certain cortico-caudate connections. Brain Res 139:43–63
Yeterian EH, Pandya DN (1993) Striatal connections of the parietal association cortices in rhesus monkeys. J Comp Neurol 332:175–197
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
François, C., Yelnik, J., Percheron, G. et al. Topographic distribution of the axonal endings from the sensorimotor and associative striatum in the macaque pallidum and substantia nigra. Exp Brain Res 102, 305–318 (1994). https://doi.org/10.1007/BF00227517
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00227517