Summary
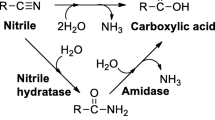
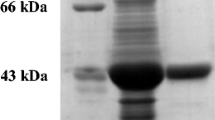
Rhodococcus rhodochrous PA-34 isolated from soil as a propionitrile-utilizing microorganism, hydrolysed several α-aminonitriles to optically active amino acids. The hydrolysis of α-aminonitriles was found to be catalysed by a nitrilase. The characteristics of the purified enzyme revealed that this is a new nitrilase as it has a molecular mass of 45 kDa and acts as a monomer. The optimum pH and temperature for the activity of the purified enzyme were 7.5 and 35° C, respectively. Thiol-specific reagents caused inhibition whereas chelators did not significantly alter the activity of this enzyme. The amino acids produced were of L-form, except for alanine. In the case of leucine production from α-aminoisocapronitrile, the enantiomeric ratio of L-leucine to D-leucine was about 60.
Similar content being viewed by others
References
Arnaud A, Galzy P, Jallageas J (1980) Production d'acides α-amines stereospecifiques par hydrolyse biolofique d'α-aminonitriles racemiques. Bull Soc Chim Fr 2:87–90
Asano Y, Fujishiro K, Tani Y, Yamada H (1982a) Aliphatic nitrile hydratase from Arthrobacter sp. J-1: purification and characterization. Agric Biol Chem 46:1165–1174
Asano Y, Tachibana M, Tani Y, Yamada H (1982b) Purification and characterization of amidase which participates in nitrile degradation. Agric Biol Chem 46:1175–1181
Asano Y, Tani Y, Yamada H (1980) A new enzyme “nitrile hydratase” which degrades acetonitrile in combination with amidase. Agric Biol Chem 44:2251–2252
Bandyopadhyay AK, Nagasawa T, Asano Y, Fujishiro K, Tani Y, Yamada H (1986) Purification and characterization of benzonitrilases from Arthrobacter sp. strain J-1. Appl Environ Microbiol 51:302–306
Bradford M (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Choi SY, Goo YM (1986) Hydrolysis of the nitrile group in α-aminophenyl acetonitrile by nitrilase; development of a new biotechnology for stereospecific production of S-α-phenylglycine. Arch Pharm Res 9:45–47
DiGeronimo MJ, Antonie AD (1976) Metabolism of acetonitrile and propionitrile by Nocardia rhodochrous LL100–21. Appl Environ Microbiol 31:900–906
Fawcett JK, Scott JE (1960) A rapid and precise method for the determination of urea. J Clin Pathol 13:156–159
Fukuda Y, Fukuni M, Harada T, Izumi Y (1971) Formation of α-amino-acid from α-aminonitrile by cell suspension of a strain of Corynebacterium. J Ferment Technol 49:1011–1016
Goldlust A, Bohak Z (1989) Induction, purification, and characterization of the nitrilase of Fusarium oxysporum f. sp. melonis. Biotechnol Appl Biochem 11:581–601
Harper DB (1977a) Microbiol metabolism of aromatic nitriles. Biochem J 165:309–319
Harper DB (1977b) Fungal degradation of aromatic nitriles. Biochem J 165:685–692
Harper DB (1985) Characterization of a nitrilase from Nocardia sp. (rhodochrous group) NCIB 11215, using p-hydroxybenzonitrile as sole carbon source. Int J Biochem 17:677–683
Hook RH, Robinson WG (1964) Ricinine nitrilase. II Purification and product. J Bio Chem 239:4263–4267
Kobayashi M, Nagasawa T, Yamada H (1989) Nitrilase of Rhodococcus rhodochrous J1: purification and characterization. Eur J Biochem 182:349–356
Kobayashi M, Yanaka N, Nagasawa T, Yamada H (1990) Purification and characterization of a novel nitrilase of Rhodococcus rhodochrous K22 that acts on aliphatic nitriles. J Bacteriol 172:4807–4815
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Macadam AM, Knowles CJ (1985) The stereospecific bioconversion of α-aminonitrile to L-alanine by an immobilized bacterium isolated from soil. Biotechno Lett 7:865–870
Nagasawa T, Takeuchi K, Yamada H (1988) Occurrence of a cobalt-induced and cobalt containing nitrile hydratase in Rhodococcus rhodochrous J1. Biochem Biophys Res Commun 155:1008–1016
Sih CJ, Fujimoto Y, Chen C (1982) Quantitative analyses of biochemical kinetic resolutions of enantiomers. J Am Chem Soc 104:7294–7299
Stalker DM, Malyj LD, McBride KE (1988) Purification and properties of a nitrilase specific for herbicide bromoxynil and corresponding nucleotide sequence analysis of bxn gene. J Biol Chem 263:6310–6314
Yamada H, Ryuno K, Nagasawa T, Enomoto K, Watanabe H (1986) Optimum culture conditions for production by Pseudomonas chlororaphis B23 of nitrile hydratase. Agric Biol Chem 50:2859–2865
Yamamoto K, Komatsu K (1991) Purification and characterization of nitrilase responsible for the enantioselective hydroylsis from Acinetobacter sp. AK 226. Agric Biol Chem 55:1459–1466
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bhalla, T.C., Miura, A., Wakamoto, A. et al. Asymmetric hydrolysis of α-aminonitriles to optically active amino acids by a nitrilase of Rhodococcus rhodochrous PA-34. Appl Microbiol Biotechnol 37, 184–190 (1992). https://doi.org/10.1007/BF00178168
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00178168