Abstract
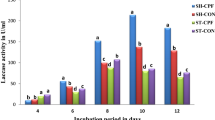
Lignin peroxidase production by the white-rot fungus Phanerochaete chrysosporium is markedly influenced by the buffer system employed. In immobilized P. chrysosporium cultures with carbon-limited glucose medium, the use of acetate buffer resulted in higher lignin peroxidase activities than tartrate. With acetate as the buffer in shake-flask cultures a 20% to over 100% improvement in lignin peroxidase production was obtained as compared to tartrate-buffered systems. Of trace elements, Cu2+, Mn2+ and Zn2+ seemed to have the greatest influence on lignin peroxidase production. Furthermore, an increase in the Cu2+ and Zn2+ concentrations resulted in considerably higher ligninase activities. Although it has been shown previously that high manganese levels repress ligninase production, for maximum ligninase production the presence of some Mn2+ appeared to be necessary. The concentration of phosphorus had surprisingly little effect on ligninase production. Highest lignin peroxidase activities were obtained with lower phosphorus concentrations, but reasonably high activities were obtained within the whole studied phosphorus range of 0.12–4.60 g l−1. Diammonium tartrate alone was a better nitrogen source than a mixture of diammonium tartrate, proteose peptone and yeast extract. The addition of solid manganese (IV) oxide to 3-day-old immobilized biocatalyst cultures increased the maximum ligninase activity obtained by about one-third.
Similar content being viewed by others
References
Asther M, Corrieu G, Drapron R, Odier E (1987) Effect of Tween 80 and oleic acid on ligninase production by Phanerochaete chrysosporium INA-12. Enzyme Microb Technol 9:245–249
Bonnarme P, Jeffries TW (1990) Mn(II) regulation of lignin peroxidases and manganese-dependent peroxidases from lignin-degrading white rot fungi. Appl Environ Microbiol 56:210–217
Capdevila C, Moukha S, Ghyczy M, Theilleux J, Gelie B, Delattre M, Corrieu G, Asther M (1990) Characterization of peroxidase secretion and subcellular organization of Phanerochaete chrysosporium INA-12 in the presence of various soybean phospholipid fractions. Appl Environ Microbiol 56:3811–3816
Glenn JK, Morgan MA, Mayfield MB, Kuwahara M, Gold MH (1983) An extracellular H2O2-requiring enzyme preparation involved in lignin biodegradation by the white-rot fungi basidiomycete Phanerochaete chrysosporium. Biochem Biophys Res Commun 114:1077–1083
Janshekar H, Fiechter A (1988) Cultivation of Phanerochaete chrysosporium and production of lignin peroxidase in submerged stirred tank reactors. J Biotechnol 8:97–112
Jeffries TW, Choi S, Kirk TK (1981) Nutritional regulation of lignin degradation by Phanerochaete chrysosporium. Appl Environ Microbiol 42:290–296
Kern HW (1989) Improvement in the production of extracellular lignin peroxidases by Phanerochaete chrysosporium: effect of solid manganese(IV)oxide. Appl Microbiol Biotechnol 32:223–234
Kern HW (1990) Production and stability of lignin peroxidases of Phanerochaete chrysosporium cultivated on glycerol in the presence of solid manganese(IV)oxide. Appl Microbiol Biotechnol 33:582–588
Kirk TK, Schultz E, Connors WJ, Lorenz LI, Zeikus JG (1978) Influence of culture parameters on lignin metabolism by Phanerochaete chrysosporium. Arch Microbiol 117:277–285
Kirk TK, Croan S, Tien M (1986) Production of multiple ligninases by Phanerochaete chrysosporium: effect of selected growth conditions and use of a mutant strain. Enzyme Microb Technol 8:27–32
Leatham GF (1986) The ligninolytic activities of Lentinus edodes and Phanerochaete chrysosporium. Appl Microbiol Biotechnol 24:51–58
Linko S (1988) Production and characterization of extracellular lignin peroxidases from immobilized Phanerochaete chrysosporium in a 10–1 bioreactor. Enzyme Microb Biotechnol 10:410–417
Linko S (1991) Production of lignin peroxidase by immobilized Phanerochaete chrysosporium. Ph.D. Thesis, Helsinki University of Technology, Technical Biochem Report 1/1991, TKK Offset, Espoo, Finland, 84 p
Linko S (1992) Production of Phanerochaete chrysosporium lignin peroxidase. Biotechnol Adv 10:191–236
Linko S, Haapala R (1993) A critical study of lignin peroxidase activity assay by veratryl alcohol oxidation. Biotechnol Tech 7:75–80
Linko S, Zhong L-C, Leisola M, Linko Y-Y, Fiechter A, Linko P (1987) Lignin peroxidase production by immobilized Phanerochaete chrysosporium in repeated batch shake cultures. In: Odier E (ed) Lignin enzymic and microbial degradation. INRA, Versailles, pp 209–213
Ma D, Hattori T, Shimada M, Higuchi T (1990) Improvement of lignin peroxidase production by Phanerochaete chrysosporium in shaking culture in the presence of polyurethane foam cubes. Wood Res 77:35–41
Michel FC, Grulke EA, Reddy CA (1990) Development of a stirred tank reactor system for production of lignin peroxidases (ligninases) by Phanerochaete chrysosporium BKM-F-1767. J Ind Microbiol 5:103–112
Michel FC, Dass SB, Grulke EA, Reddy CA (1991) Role of manganese peroxidases and lignin peroxidases of Phanerochaete chrysosporium in the decolorization of Kraft bleach plant effluent. Appl Environ Microbiol 57:2368–2375
Nelson NA (1944) A photometric adaption of the Somogyi method for determination of glucose. J Biol Chem 153:375–380
Pan CH, Hepler L, Elander RP (1975) The effect of iron on a high-yielding industrial strain of Penicillium chrysogenum and production levels of penicillin G. J Ferment Technol 53:854–861
Perez J, Jeffries TW (1990) Mineralization of 14C-ring-labeled synthetic lignin correlates with the production of lignin peroxidase, not of manganese peroxidase or laccase. Appl Environ Microbiol 56:1806–1812
Reid ID (1983) Effects of nitrogen supplements on degradation of aspen wood lignin and carbohydrate components by Phanerochaete chrysosporium. Appl Environ Microbiol 45:830–837
Renganathan V, Miki K, Gold MH (1985) Multiple molecular forms of diarylpropane oxygenase, and H2O2-requiring, lignin-degrading enzyme from Phanerochaete chrysosporium. Arch Biochem Biophys 241:304–314
Roch P, Buswell JA, Cain RB, Odier E (1989) Lignin peroxidase production by strains of Phanerochaete chrysosporium grown on glycerol. Appl Microbiol Biotechnol 31:587–591
Tien M, Kirk TK (1983) Lignin-degrading enzyme from hymenomycete Phanerochaete chrysosporium Burds. Science 221:661–663
Author information
Authors and Affiliations
Additional information
Correspondence to: S. Linko
Rights and permissions
About this article
Cite this article
Haapala, R., Linko, S. Production of Phanerochaete chrysosporium lignin peroxidase under various culture conditions. Appl Microbiol Biotechnol 40, 494–498 (1993). https://doi.org/10.1007/BF00175737
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00175737