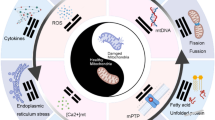
Abstract
Mitochondria, as the “powerhouse” of eukaryotic cells, play the key role in central signalling pathways decisive for the cell fate (proliferation, differentiation, growth and death) as well as systemic events and effects including stress response towards environmental changes, redox balance, the innate and acquired immunity as well as severity of the acute and chronic disorders. Up to now no any health condition has been reported which mitochondrial functionality would be irrelevant for. Moreover, accumulated research data demonstrate that aside from the energetic aspects which are decisive for the health and death at the sub/cellular, tissue, organ and organismal levels, injured mitochondria do release specific damage-associated molecular patters. To this end, cell-free mitochondrial (mtDNA) fragments are recognised as the “mitochondrial burnout” signals triggering systemic effects such as non-infectious (sterile) inflammation, which are further involved in pathomechanisms of downstream diseases. Well-known mitochondrial burnout-associated pathologies include chronic fatigue, accelerated ageing, auto/immune disorders, hormonal dysregulation and infertility, eye pathologies, metabolic and mood disorders, severe respiratory diseases, impaired healing, neurodegenerative and cancerous alterations. There is an evident reciprocity between mitochondrial and organismal health status: compromised mitochondrial health is reflected in systemic damage as well as organismal health-to-disease transition is reflected in an altered mitochondrial signalling. Contextually, mitochondrion acts as a natural biosensor integrated into human cells, and the routine non-invasive mitochondrial health quality control test is a powerful tool for the holistic predictive diagnostic approach in PPPM-framework highly recommended at the level of primary and secondary care for
-
the whole-body health quality check-up,
-
pre-pregnancy check-up,
-
health-to-disease transition check-up,
-
accompanying diagnostics in sport medicine and supervised physical activities,
-
accompanying diagnostics in physiotherapeutic and well-being services,
-
therapy efficacy monitoring for personalised treatments (e.g., chronic fatigue; burnout syndrome and sleep disorders; eye, skin, kidney, liver and respiratory diseases, endocrine and cardiovascular impairments, musculoskeletal- and neuro-degenerative disorders, depression, etc.).
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Abbreviations
- 3PM:
-
Predictive, preventive and personalised medicine
- AD:
-
Atopic dermatitis
- AF:
-
Atrial fibrillation
- ATP:
-
Adenosine triphosphate
- BHI:
-
Bioenergetics health index
- BRB:
-
Blood–retinal barrier
- CFS:
-
Chronic fatigue syndrome
- cGAS/STING:
-
Cyclic GMP-AMP Synthase/Stimulator of Interferon Genes
- COVID:
-
Corona virus disease
- CTD:
-
Connective tissue dysregulation
- DAKD:
-
Diabetes associated kidney disease
- DM:
-
Diabetes mellitus
- DR:
-
Diabetic retinopathy
- ECM:
-
Extracellular matrix
- ESRD:
-
End stage renal disease
- FSP:
-
Flammer syndrome phenotype
- HIF1-alpha:
-
Hypoxia inducible factor 1 alpha subunit
- IL-18:
-
Interleukin 18
- IL-1β:
-
Interleukin 1β
- IS:
-
Ischemic stroke
- MAVS:
-
Mitochondria-associated adaptor molecule
- MHI:
-
Mitochondrial health index
- MHQC:
-
Mitochondrial health quality control
- MIA:
-
Maternal immune activation
- MRI:
-
Magnetic resonance imaging
- mtDNA:
-
Mitochondrial Deoxyribonucleic acid
- mtDNA-CN:
-
Mitochondrial deoxyribonucleic acid- copy number
- NLRP3:
-
Nucleotide-binding domain, Leucine-Rich–containing family, Pyrin domain-containing-3 protein
- NRF2:
-
Nuclear factor erythroid 2–related factor 2
- OSAS:
-
Obstructive sleep apnoea syndrome
- PDR:
-
Proliferative diabetic retinopathy
- PPPM:
-
Predictive, preventive and personalised medicine
- RMEC:
-
Retinal microvascular endothelial cells
- ROS:
-
Reactive oxygen species
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- TLR9:
-
Toll-like receptor 9
- ZBP1:
-
Z-DNA binding protein 1
References
Wu Z, Sainz AG, Shadel GS (2021) Mitochondrial DNA: cellular genotoxic stress sentinel. Trends Biochem Sci 46(10):812–821. https://doi.org/10.1016/j.tibs.2021.05.004
Vasan K, Werner M, Chandel NS (2020) Mitochondrial metabolism as a target for cancer therapy. Cell Metab 32(3):341–352. https://doi.org/10.1016/j.cmet.2020.06.019
Picard M, McEwen BS (2018) Psychological stress and mitochondria: a conceptual framework. Psychosom Med 80(2):126–140. https://doi.org/10.1097/PSY.0000000000000544
Fernandez-Vizarra E, Zeviani M (2021) Mitochondrial disorders of the OXPHOS system. FEBS Lett 595(8):1062–1106. https://doi.org/10.1002/1873-3468.13995
Koklesova L, Mazurakova A, Samec M, Kudela E, Biringer K, Kubatka P, Golubnitschaja O (2022) Mitochondrial health quality control: measurements and interpretation in the framework of predictive. EPMA J 13(2):177–193. https://doi.org/10.1007/s13167-022-00281-6
Whitley BN, Engelhart EA, Hoppins S (2019) Mitochondrial dynamics and their potential as a therapeutic target. Mitochondrion 49:269–283. https://doi.org/10.1016/j.mito.2019.06.002
de Goede P, Wefers J, Brombacher EC, Schrauwen P, Kalsbeek A (2018) Circadian rhythms in mitochondrial respiration. J Mol Endocrinol 60(3):R115–R130. https://doi.org/10.1530/JME-17-0196
Bescos R, Boden MJ, Jackson ML, Trewin AJ, Marin EC, Levinger I, Garnham A, Hiam DS, Falcao-Tebas F, Conte F, Owens JA, Kennaway DJ, McConell GK (2018) Four days of simulated shift work reduces insulin sensitivity in humans. Acta Physiol (Oxf) 223(2):e13039. https://doi.org/10.1111/apha.13039
Rabinovich-Nikitin I, Kirshenbaum LA (2022) Circadian regulated control of myocardial ischemia-reperfusion injury. Trends Cardiovasc Med. https://doi.org/10.1016/j.tcm.2022.09.003
Munmun F, Witt-Enderby PA (2021) Melatonin effects on bone: implications for use as a therapy for managing bone loss. J Pineal Res 71(1):e12749. https://doi.org/10.1111/jpi.12749
Ahluwalia A, Patel K, Hoa N, Brzozowska I, Jones MK, Tarnawski AS (2021) Melatonin ameliorates aging-related impaired angiogenesis in gastric endothelial cells via local actions on mitochondria and VEGF-survivin signaling. Am J Physiol Gastrointest Liver Physiol 321(6):G682–G689. https://doi.org/10.1152/ajpgi.00101.2021
Mostafalou S, Abdollahi M (2013) Pesticides and human chronic diseases: evidences, mechanisms, and perspectives. Toxicol Appl Pharmacol 268(2):157–177. https://doi.org/10.1016/j.taap.2013.01.025
Chao T, Shih HT, Hsu SC, Chen PJ, Fan YS, Jeng YM, Shen ZQ, Tsai TF, Chang ZF (2021) Autophagy restricts mitochondrial DNA damage-induced release of ENDOG (endonuclease G) to regulate genome stability. Autophagy 17(11):3444–3460. https://doi.org/10.1080/15548627.2021.1874209
Picard M, McEwen BS, Epel ES, Sandi C (2018) An energetic view of stress: focus on mitochondria. Front Neuroendocrinol 49:72–85. https://doi.org/10.1016/j.yfrne.2018.01.001
Méthot SJ, Proulx S, Brunette I, Rochette PJ (2020) Chronology of cellular events related to mitochondrial burnout leading to cell death in Fuchs endothelial corneal dystrophy. Sci Rep 10(1):5811. https://doi.org/10.1038/s41598-020-62602-x
Armstrong CW, McGregor NR, Butt HL, Gooley PR (2014) Metabolism in chronic fatigue syndrome. Adv Clin Chem 66:121–172. https://doi.org/10.1016/b978-0-12-801401-1.00005-0
Gorman GS, Elson JL, Newman J, Payne B, McFarland R, Newton JL, Turnbull DM (2015) Perceived fatigue in highly prevalent and debilitating in patients with mitochondrial disease. Neuromuscul Disord 25(7):563–566. https://doi.org/10.1016/j.nmd.2015.03.001
Anderson G, Maes M (2020) Mitochondria and immunity in chronic fatigue syndrome. Prog Neuropsychopharmacol Biol Psychiatry 103:109976. https://doi.org/10.1016/j.pnpbp.2020.109976
Ohba T, Domoto S, Tanaka M, Nakamura S, Shimazawa M, Hara H (2019) Myalgic encephalomyelitis/chronic fatigue syndrome induced by repeated forced swimming in mice. Biol Pharm Bull 42(7):1140–1145. https://doi.org/10.1248/bpb.b19-00009
Youle RJ, van der Bliek AM (2012) Mitochondrial fission, fusion, and stress. Science 337(6098):1062–1065. https://doi.org/10.1126/science.1219855
Barrera MJ, Aguilera S, Castro I, Carvajal P, Jara D, Molina C, González S, González MJ (2021) Dysfunctional mitochondria as critical players in the inflammation of autoimmune diseases: potential role in Sjögren’s syndrome. Autoimmun Rev 20(8):102867. https://doi.org/10.1016/j.autrev.2021.102867
Konieczka K, Ritch R, Traverso CE, Kim DM, Kook MS, Gallino A, Golubnitschaja O, Erb C, Reitsamer HA, Kida T, Kurysheva N, Yao K (2014) Flammer syndrome. EPMA J 5(1):11. https://doi.org/10.1186/1878-5085-5-11
Golubnitschaja O (ed) (2019) Flammer syndrome—from phenotype to associated pathologies, prediction, prevention and personalisation V.11. ISBN 978-3-030-13549-2 ISBN 978-3-030-13550-8 (eBook). https://doi.org/10.1007/978-3-030-13550-8
Evsevieva M, Sergeeva O, Mazurakova A, Koklesova L, Prokhorenko-Kolomoytseva I, Shchetinin E, Birkenbihl C, Costigliola V, Kubatka P, Golubnitschaja O (2022) Pre-pregnancy check-up of maternal vascular status and associated phenotype is crucial for the health of mother and offspring. EPMA J 13(3):351. https://doi.org/10.1007/s13167-022-00294-1
Liskova A, Samec M, Koklesova L, Kudela E, Kubatka P, Golubnitschaja O (2021) Mitochondriopathies as a clue to systemic disorders - analytical tools and mitigating measures in context of predictive, preventive, and personalized (3P) medicine. IJMS 22(4):2007. https://doi.org/10.3390/ijms22042007
Koklesova L, Samec M, Liskova A, Zhai K, Büsselberg D, Giordano FA, Kubatka P, Golunitschaja O (2021) Mitochondrial impairments in aetiopathology of multifactorial diseases: common origin but individual outcomes in context of 3P medicine. EPMA J 12(1):27–40. https://doi.org/10.1007/s13167-021-00237-2
Torres Crigna A, Link B, Samec M, Giordano FA, Kubatka P, Golubnitschaja O (2021) Endothelin-1 axes in the framework of predictive, preventive and personalised (3P) medicine. EPMA J 12(3):1–41. https://doi.org/10.1007/s13167-021-00248-z
Golubnitschaja O, Liskova A, Koklesova L, Samec M, Biringer K, Büsselberg D, Podbielska H, Kunin AA, Evsevyeva ME, Shapira N, Paul F, Erb C, Dietrich DE, Felbel D, Karabatsiakis A, Bubnov R, Polivka J, Polivka J Jr, Birkenbihl C, Fröhlich H, Hofmann-Apitius M, Kubatka P (2021) Caution, “normal” BMI: health risks associated with potentially masked individual underweight EPMA position paper 2021. EPMA J 12(3):1–22. https://doi.org/10.1007/s13167-021-00251-4
3Pmedicon. https://3pmedicon.de/en/
Golubnitschaja O, Potuznik P, Polivka J Jr, Pesta M, Kaverina O, Pieper CC, Kropp M, Thumann G, Erb C, Karabatsiakis A, Stetkarova I, Polivka J, Costigliola V (2022) Ischemic stroke of unclear aetiology: a case-by-case analysis and call for a multi-professional predictive, preventive and personalised approach. EPMA J 13(4):535–545. https://doi.org/10.1007/s13167-022-00307-z
Amorim JA, Coppotelli G, Rolo AP, Palmeira CM, Ross JM, Sinclair DA (2022) Mitochondrial and metabolic dysfunction in ageing and age-related diseases. Nat Rev Endocrinol 18(4):243–258. https://doi.org/10.1038/s41574-021-00626-7
van der Reest J, Cecchino GN, Haigis MC, Kordowitzki P (2021) Mitochondria: their relevance during oocyte ageing. Ageing Res Rev 70:101378. https://doi.org/10.1016/j.arr.2021.101378
Boguenet M, Bouet PE, Spiers A, Reynier P, May-Panloup P (2021) Mitochondria: their role in spermatozoa and in male infertility. Hum Reprod Update 27(4):697–719. https://doi.org/10.1093/humupd/dmab001
Masoudi R, Asadzadeh N, Sharafi M (2021) Effects of freezing extender supplementation with mitochondria-targeted antioxidant Mito-TEMPO on frozen-thawed rooster semen quality and reproductive performance. Anim Reprod Sci 225:106671. https://doi.org/10.1016/j.anireprosci.2020.106671
Shaw GA (2021) Mitochondria as the target for disease related hormonal dysregulation. Brain Behav Immun Health 18:100350. https://doi.org/10.1016/j.bbih.2021.100350
Gyllenhammer LE, Rasmussen JM, Bertele N, Halbing A, Entringer S, Wadhwa PD, Buss C (2022) Maternal inflammation during pregnancy and offspring brain development: the role of mitochondria. Biol Psychiatry Cogn Neurosci Neuroimaging 7(5):498–509. https://doi.org/10.1016/j.bpsc.2021.11.003
Schaefer PM, Scherer Alves L, Lvova M, Huang J, Rathi K, Janssen K, Butic A, Yardeni T, Morrow R, Lott M, Murdock D, Song A, Keller K, Garcia BA, Francomano CA, Wallace DC (2022) Combination of common mtDNA variants results in mitochondrial dysfunction and a connective tissue dysregulation. Proc Natl Acad Sci U S A 119(45):e2212417119. https://doi.org/10.1073/pnas.2212417119
Effendi WI, Nagano T (2022) Connective tissue growth factor in idiopathic pulmonary fibrosis: breaking the bridge. Int J Mol Sci 23(11):6064. https://doi.org/10.3390/ijms23116064
Nelson KK, Melendez JA (2004) Mitochondrial redox control of matrix metalloproteinases. Free Radic Biol Med 37(6):768–784. https://doi.org/10.1016/j.freeradbiomed.2004.06.008
Pool L, Wijdeveld LFJM, de Groot NMS, Brundel BJJM (2021) The role of mitochondrial dysfunction in atrial fibrillation: translation to Druggable target and biomarker discovery. Int J Mol Sci 22:8463. https://doi.org/10.3390/ijms22168463
Yang J-L, Mukda S, Chen S-D (2018) Diverse roles of mitochondria in ischemic stroke. Redox Biol 16:263–275. https://doi.org/10.1016/j.redox.2018.03.002
Ham PB, Raju R (2017) Mitochondrial function in hypoxic ischemic injury and influence of aging. Prog Neurobiol 157:92–116. https://doi.org/10.1016/j.pneurobio.2016.06.006
Anzell AR, Maizy R, Przyklenk K, Sanderson TH (2018) Mitochondrial quality control and disease: insights into ischemia-reperfusion injury. Mol Neurobiol 55:2547–2564. https://doi.org/10.1007/s12035-017-0503-9
He Z, Ning N, Zhou Q, Khoshnam SE, Farzaneh M (2020) Mitochondria as a therapeutic target for ischemic stroke. Free Radic Biol Med 146:45–58. https://doi.org/10.1016/j.freeradbiomed.2019.11.005
Teng Z, Dong Y, Zhang D, An J, Lv P (2017) Cerebral small vessel disease and post-stroke cognitive impairment. Int J Neurosci 127:824–830. https://doi.org/10.1080/00207454.2016.1261291
Nahirney PC, Reeson P, Brown CE (2016) Ultrastructural analysis of blood-brain barrier breakdown in the peri-infarct zone in young adult and aged mice. J Cereb Blood Flow Metab 36:413–425. https://doi.org/10.1177/0271678X15608396
Slominski AT, Zmijewski MA, Semak I, Kim TK, Janjetovic Z, Slominski RM, Zmijewski JW (2017) Melatonin, mitochondria, and the skin. Cell Mol Life Sci 74(21):3913–3925. https://doi.org/10.1007/s00018-017-2617-7
Sreedhar A, Aguilera-Aguirre L, Singh KK (2020) Mitochondria in skin health, aging, and disease. Cell Death Dis 11(6):444. https://doi.org/10.1038/s41419-020-2649-z
Koch M, Kockmann T, Rodriguez E, Wehkamp U, Hiebert P, Ben-Yehuda Greenwald M, Stölzl D, Beer HD, Tschachler E, Weidinger S, Werner S, Auf dem Keller U (2023) Quantitative proteomics identifies reduced NRF2 activity and mitochondrial dysfunction in atopic dermatitis. J Invest Dermatol 143(2):220–231.e7. https://doi.org/10.1016/j.jid.2022.08.048
Leman G, Pavel P, Hermann M, Crumrine D, Elias PM, Minzaghi D, Goudounèche D, Roshardt Prieto NM, Cavinato M, Wanner A, Blunder S, Gruber R, Jansen-Dürr P, Dubrac S (2022) Mitochondrial activity is upregulated in nonlesional atopic dermatitis and amenable to therapeutic intervention. J Invest Dermatol 142(10):2623–2634.e12. https://doi.org/10.1016/j.jid.2022.01.035
Kim MJ, Kim SN, Lee YW, Choe YB, Ahn KJ (2016) Vitamin D status and efficacy of vitamin D supplementation in atopic dermatitis: a systematic review and meta-analysis. Nutrients 8(12):789. https://doi.org/10.3390/nu8120789
Ashcroft SP, Fletcher G, Philp AM, Jenkinson C, Das S, Hansbro PM, Atherton PJ, Philp A (2021) Diet-induced vitamin D deficiency reduces skeletal muscle mitochondrial respiration. J Endocrinol 249(2):113–124. https://doi.org/10.1530/JOE-20-0233
Willenborg S, Sanin DE, Jais A, Ding X, Ulas T, Nüchel J, Popović M, MacVicar T, Langer T, Schultze JL, Gerbaulet A, Roers A, Pearce EJ, Brüning JC, Trifunovic A, Eming SA (2021) Mitochondrial metabolism coordinates stage-specific repair processes in macrophages during wound healing. Cell Metab 33(12):2398–2414.e9. https://doi.org/10.1016/j.cmet.2021.10.004
Sanchez MC, Lancel S, Boulanger E, Neviere R (2018) Targeting oxidative stress and mitochondrial dysfunction in the treatment of impaired wound healing: a systematic review. Antioxidants (Basel) 7(8):98. https://doi.org/10.3390/antiox7080098
Javad F, Day PJ (2012) Protein profiling of keloidal scar tissue. Arch Dermatol Res 304(7):533–540. https://doi.org/10.1007/s00403-012-1224-6
Luo Y, Ma J, Lu W (2020) The significance of mitochondrial dysfunction in cancer. Int J Mol Sci 21(16):5598. https://doi.org/10.3390/ijms21165598
Memon AA, Vats S, Sundquist J, Li Y, Sundquist K (2022) Mitochondrial DNA copy number: linking diabetes and cancer. Antioxid Redox Signal 37(16–18):1168–1190. https://doi.org/10.1089/ars.2022.0100
Srinivasan S, Guha M, Kashina A, Avadhani NG (2017) Mitochondrial dysfunction and mitochondrial dynamics-the cancer connection. Biochim Biophys Acta Bioenerg 1858(8):602–614. https://doi.org/10.1016/j.bbabio.2017.01.004
Filograna R, Mennuni M, Alsina D, Larsson NG (2021) Mitochondrial DNA copy number in human disease: the more the better? FEBS Lett 595(8):976–1002. https://doi.org/10.1002/1873-3468.14021
Brunetti V, Della Marca G, Servidei S, Primiano G (2021) Sleep disorders in mitochondrial diseases. Curr Neurol Neurosci Rep 21(7):30. https://doi.org/10.1007/s11910-021-01121-2
Lacedonia D, Carpagnano GE, Crisetti E, Cotugno G, Palladino GP, Patricelli G, Sabato R, Foschino Barbaro MP (2015) Mitochondrial DNA alteration in obstructive sleep apnea. Respir Res 16(1):47. https://doi.org/10.1186/s12931-015-0205-7
Beaupre LMM, Brown GM, Braganza NA, Kennedy JL, Gonçalves VF (2022) Mitochondria’s role in sleep: novel insights from sleep deprivation and restriction studies. World J Biol Psychiatry 23(1):1–13. https://doi.org/10.1080/15622975.2021.1907723
Heyat MBB, Akhtar F, Sultana A, Tumrani S, Teelhawod BN, Abbasi R, Kamal MA, Muaad AY, Lai D, Wu K (2022) Role of oxidative stress and inflammation in insomnia sleep disorder and cardiovascular diseases: herbal antioxidants and anti-inflammatory coupled with insomnia detection using machine learning. Curr Pharm Des 28:3618. https://doi.org/10.2174/1381612829666221201161636
Frau-Méndez MA, Fernández-Vega I, Ansoleaga B, Tech RB, Tech MC, Del Rio JA, Zerr I, Llorens F, Zarranz JJ, Ferrer I (2017) Fatal familial insomnia: mitochondrial and protein synthesis machinery decline in the mediodorsal thalamus. Brain Pathol 27(1):95–106. https://doi.org/10.1111/bpa.12408
Pattinson CL, Guedes VA, Edwards K, Mithani S, Yun S, Taylor P, Dunbar K, Kim HS, Chen Lai C, Roy MJ, Gill JM (2020) Excessive daytime sleepiness is associated with altered gene expression in military personnel ad veterans with posttraumatic stress disorder: an RNA sequencing study. Sleep 43(9):zsaa036. https://doi.org/10.1093/sleep/zsaa036
Kim Y, Vadodaria KC, Lenkei Z, Kato T, Gage FH, Marchetto MC, Santos R (2019) Mitochondria, metabolism, and redox mechanisms in psychiatric disorders. Antioxid Redox Signal 31(4):275–317. https://doi.org/10.1089/ars.2018.7606
Hollis F, Pope BS, Gorman-Sandler E, Wood SK (2022) Neuroinflammation and mitochondrial dysfunction Link social stress to depression. Curr Top Behav Neurosci 54:59–93. https://doi.org/10.1007/7854_2021_300
Tripathi A, Scaini G, Barichello T, Quevedo J, Pillai A (2021) Mitophagy in depression: pathophysiology and treatment targets. Mitochondrion 61:1–10. https://doi.org/10.1016/j.mito.2021.08.016
Bansal Y, Kuhad A (2016) Mitochondrial dysfunction in depression. Curr Neuropharmacol 4(6):610–618. https://doi.org/10.2174/1570159x14666160229114755
Chow J, Rahman J, Achermann JC, Dattani MT, Rahman S (2017) Mitochondrial disease and endocrine dysfunction. Nat Rev Endocrinol 13(2):92–104. https://doi.org/10.1038/nrendo.2016.151
Duann P, Lin PH (2017) Mitochondria damage and kidney disease. Adv Exp Med Biol 982:529–551. https://doi.org/10.1007/978-3-319-55330-6_27
Ahmad AA, Draves SO, Rosca M (2021) Mitochondria in diabetic kidney disease. Cell 10(11):2945. https://doi.org/10.3390/cells10112945
Ma X, McKeen T, Zhang J, Ding WX (2020) Role and mechanisms of mitophagy in liver diseases. Cell 9(4):837. https://doi.org/10.3390/cells9040837
Zhang H, Yan Q, Wang X, Chen X, Chen Y, Du J, Chen L (2021) The role of mitochondria in liver ischemia-reperfusion injury: from aspects of mitochondrial oxidative stress, mitochondrial fission, mitochondrial membrane permeable transport pore formation, mitophagy, and mitochondria-related protective measures. Oxid Med Cell Longev 2021:6670579. https://doi.org/10.1155/2021/6670579
Guo Y, Gu R, Gan D, Hu F, Li G, Xu G (2020) Mitochondrial DNA drives noncanonical inflammation activation via cGAS-STING signaling pathway in retinal microvascular endothelial cells. Cell Commun Signal 18(1):172. https://doi.org/10.1186/s12964-020-00637-3
Grytz R, Yang H, Hua Y, Samuels BC, Sigal IA (2020) Connective tissue remodeling in myopia and its potential role in increasing risk of glaucoma. Curr Opin Biomed Eng 15:40–50. https://doi.org/10.1016/j.cobme.2020.01.001
Jassim AH, Inman DM, Mitchell CH (2021) Crosstalk between dysfunctional mitochondria and inflammation in glaucomatous neurodegeneration. Front Pharmacol 12:699623. https://doi.org/10.3389/fphar.2021.699623
Jassim AH, Fan Y, Pappenhagen N, Nsiah NY, Inman DM (2021) Oxidative stress and hypoxia modify mitochondrial homeostasis during glaucoma. Antioxid Redox Signal 35(16):1341–1357. https://doi.org/10.1089/ars.2020.8180
Golubnitschaja O, Flammer J (2007) What are the biomarkers for glaucoma? Surv Ophthalmol 52(Suppl 2):S155–S161. https://doi.org/10.1016/j.survophthal.2007.08.011
Golubnitschaja O, Yeghiazaryan K, Flammer J (2010) Key molecular pathways affected by glaucoma pathology: is predictive diagnosis possible? EPMA J 1(2):237–244. https://doi.org/10.1007/s13167-010-0031-4
Yeghiazaryan K, Flammer J, Orgül S, Wunderlich K, Golubnitschaja O (2009) Vasospastic individuals demonstrate significant similarity to glaucoma patients as revealed by gene expression profiling in circulating leukocytes. Mol Vis 15:2339–2348
Golubnitschaja O (2018) The keyrole of multiomics in the predictive, preventive and personalised medical approach towards glaucoma management. Klin Monbl Augenheilkd 235(2):146–150. https://doi.org/10.1055/s-0044-101164
Zhan X, Li J, Guo Y, Golubnitschaja O (2021) Mass spectrometry analysis of human tear fluid biomarkers specific for ocular and systemic diseases in the context of 3P medicine. EPMA J 12(4):449–475. https://doi.org/10.1007/s13167-021-00265-y
Kropp M, Golubnitschaja O, Mazurakova A, Koklesova L, Sargheini N, Vo T-TKS, de Clerck E, Polivka J, Potuznik P, Polivka J, Stetkarova I, Kubatka P, Thumann G (2023) Diabetic retinopathy as the leading cause of blindness and early predictor of cascading complications—risks and mitigation. EPMA J 14(1):21. https://doi.org/10.1007/s13167-023-00314-8
Wu H, Yu Y, David L, Ho Y-S, Lou MF (2014) Glutaredoxin 2 (Grx2) gene deletion induces early onset of age-dependent cataracts in mice. J Biol Chem 289(52):36125–36139. https://doi.org/10.1074/jbc.M114.620047
Horga A, Bugiardini E, Manole A, Bremner F, Jaunmuktane Z, Dankwa L, Rebelo AP, Woodward CE, Hargreaves IP, Cortese A, Pittman AM, Brandner S, Polke JM, Pitceathly RDS, Züchner S, Hanna MG, Scherer SS, Houlden H, Reilly MM (2019) Autosomal dominant optic atrophy and cataract “plus” phenotype including axonal neuropathy. Neurol Genet 5(2):e322. https://doi.org/10.1212/NXG.0000000000000322
Pugazhenthi S, Qin L, Reddy PH (2017) Common neurodegenerative pathways in obesity, diabetes, and Alzheimer’s disease. Biochim Biophys Acta Mol Basis Dis 1863(5):1037–1045. https://doi.org/10.1016/j.bbadis.2016.04.017
Song T, Song X, Zhu C, Patrick R, Skurla M, Santangelo I, Green M, Harper D, Ren B, Forester BP, Öngür D, Du F (2021) Mitochondrial dysfunction, oxidative stress, neuroinflammation, and metabolic alterations in the progression of Alzheimer’s disease: a meta-analysis of in vivo magnetic resonance spectroscopy studies. Ageing Res Rev 72:101503. https://doi.org/10.1016/j.arr.2021.101503
Bourebaba L, Kornicka-Garbowska K, Galuppo L, Marycz K (2022) Artificial mitochondrial transfer (AMT) for the management of age-related musculoskeletal degenerative disorders: an emerging avenue for bone and cartilage metabolism regulation. Stem Cell Rev Rep 18(6):2195–2201. https://doi.org/10.1007/s12015-022-10357-5
Prakash YS, Pabelick CM, Sieck GC (2017) Mitochondrial dysfunction in airway disease. Chest 152(3):618–626. https://doi.org/10.1016/j.chest.2017.03.020
Qian L, Mehrabi Nasab E, Athari SM, Athari SS (2022) Mitochondria signaling pathways in allergic asthma. J Invest Med 70(4):863–882. https://doi.org/10.1136/jim-2021-002098
Michaeloudes C, Abubakar-Waziri H, Lakhdar R, Raby K, Dixey P, Adcock IM, Mumby S, Bhavsar PK, Chung KF (2022) Molecular mechanisms of oxidative stress in asthma. Mol Aspects Med 85:101026. https://doi.org/10.1016/j.mam.2021.101026
Esteves P, Celle A, Berger P, Trian T (2020) Bronchial smooth muscle mitochondria: a new target for asthma therapy? Rev Mal Respir 37(3):201–204. https://doi.org/10.1016/j.rmr.2020.02.004
Wood E, Hall KH, Tate W (2021) Role of mitochondria, oxidative stress and the response to antioxidants in myalgic encephalomyelitis/chronic fatigue syndrome: a possible approach to SARS-CoV-2 ‘long-haulers’? Chronic Dis Transl Med 7(1):14–26. https://doi.org/10.1016/j.cdtm.2020.11.002
Soukas AA, Hao H, Wu L (2019) Metformin as anti-aging therapy: is it for everyone? Trends Endocrinol Metab 30(10):745–755. https://doi.org/10.1016/j.tem.2019.07.015
Cebioglu M, Schild HH, Golubnitschaja O (2010) Cancer predisposition in diabetics: risk factors considered for predictive diagnostics and targeted preventive measures. EPMA J 1(1):130–137. https://doi.org/10.1007/s13167-010-0015-4
Teresa VT (2014) Mitochondrial biogenesis: pharmacological approaches. Curr Pharm Des 20(35):5507–5509. https://doi.org/10.2174/138161282035140911142118
Sanz R, Mazzei L, Santino N, Ingrasia M, Manucha W (2020) Vitamin D-mitochondria cross-talk could modulate the signaling pathway involved in hypertension development: a translational integrative overview. Clin Investig Arterioscler 32(4):144–155. https://doi.org/10.1016/j.arteri.2020.02.002
Boyko N, Golubnitschaja O (eds) (2023) Microbiome in 3P medicine strategies—the first exploitation guide. ISSN 2211-3495 ISSN 2211-3509 (electronic) Advances in Predictive, Preventive and Personalised Medicine ISBN 978-3-031-19563-1 ISBN 978-3-031-19564-8 (eBook). https://doi.org/10.1007/978-3-031-19564-8
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Golubnitschaja, O. (2023). What Is the Routine Mitochondrial Health Check-Up Good For? A Holistic Approach in the Framework of 3P Medicine. In: Podbielska, H., Kapalla, M. (eds) Predictive, Preventive, and Personalised Medicine: From Bench to Bedside. Advances in Predictive, Preventive and Personalised Medicine, vol 17. Springer, Cham. https://doi.org/10.1007/978-3-031-34884-6_3
Download citation
DOI: https://doi.org/10.1007/978-3-031-34884-6_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-34883-9
Online ISBN: 978-3-031-34884-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)