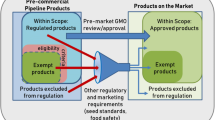
Abstract
Argentina is a world leader in regards to regulation and adoption of GM crops. As a consequence, the regulatory aspects of gene editing applied to agriculture were considered proactively, and a simple but sound pioneer regulation was developed.
At present, the Argentine regulatory system is fully able to establish if a gene-edited crop should be classified (and handled) either as a GM crop or a conventional new variety. To this end, the concept of “novel combination of genetic material” derived from the Cartagena Protocol is of paramount importance.
After some pilot cases that have been handled under the new regulation, applicants appreciate the ease, speed and predictability of this regulation. Moreover, it has been considered by other countries in develo** their own regulations, thus acting also as a harmonization factor for the safe and effective insertion of these technologies in the global market.
The information and views are those of the authors as individuals and experts in the field, and do not necessarily represent those of the organizations where they work.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Notes
- 1.
ISAAA (2016).
- 2.
ASA (2018).
- 3.
INDEC (2018).
- 4.
COMTRADE (2018).
- 5.
MINAGRO (2018).
- 6.
ISAAA (2016).
- 7.
Barfoot and Brookes (2014).
- 8.
Trigo (2016).
- 9.
Penna and Lema (2003).
- 10.
Bustamante (2018).
- 11.
Whelan and Lema (2015).
- 12.
- 13.
INFOLEG (1973).
- 14.
INFOLEG (2015).
- 15.
INFOLEG (1992). Noteworthy, there is also a National Law 20.270 on the Promotion of the Development and Production of Modern Biotechnology—see INFOLEG (2007). However, it does not play any role in regulatory aspects, since it is focused on granting funding and tax incentives to biotechnology-Based Innovative Enterprises—see Rosada (2018).
- 16.
INFOLEG (2011a).
- 17.
INFOLEG (2011b).
- 18.
WTO (2018).
- 19.
CBD (2000).
- 20.
CBD (2018a).
- 21.
CBD (2018b).
- 22.
FAO (2014a).
- 23.
SENASA (2018).
- 24.
INFOLEG (2011c).
- 25.
Ishii and Araki (2017).
- 26.
EFSA (2012).
- 27.
Lusser et al. (2011).
- 28.
WHO (2018).
- 29.
INFOLEG (2004).
- 30.
INFOLEG (2012a).
- 31.
INFOLEG (2013a).
- 32.
INFOLEG (2013b).
- 33.
INFOLEG (2002a).
- 34.
INFOLEG (2011d).
- 35.
INFOLEG (2018).
- 36.
Not to be confused with the National Register of Property of Plant varieties, which is a different register also under the National Seed Institute but devoted to the protection of intellectual property rights on a plant variety.
- 37.
Cascardo et al. (1998).
- 38.
INFOLEG (2017a).
- 39.
FAO (2014b).
- 40.
It has been argued elsewhere—see OECD (2015)—that detection of gene-edited products may be more challenging compared to GMOs currently on the market, based on reasons of both technical and human nature. However, from a technical viewpoint, even the slightest mutations obtained by gene editing can be easily detected by molecular techniques currently available, such as those applied in marker-assisted breeding programs to detect Single-Nucleotide Polymorphism (SNPs). Of course, this assertion is true as long as the mutation is known but the same is also valid for GMOs, where the introduced gene or protein must be known in order to design a detection method. In regards to “human nature”, considerations relate to the fact that gene editing may lead to mutations that could be generated spontaneously (or using physical or chemical techniques for mutagenesis); this has been presented as a potential temptation for concealing the method used for obtaining the trait. However, such covering would be very difficult in reality since biotech developers employ several people to generate such products (who should be accomplice of the plot for an indefinite time), and they forcedly leave a trace of patents, papers, presentations to regulatory frameworks of third countries and other public information.
- 41.
AOSCA (2018).
- 42.
OECD (2018).
- 43.
Schiavone et al. (2013).
- 44.
- 45.
Berto (2018).
- 46.
INFOLEG (2017b).
- 47.
FAO (2011).
- 48.
Phillips and Smyth (2002).
- 49.
FAO (2000).
- 50.
Pensel et al. (2007).
- 51.
INFOLEG (2012b).
- 52.
Valor Soja (2017).
- 53.
Co-Extra (2005).
- 54.
Ghezan and Tapia (2006).
- 55.
Ghezan et al. (2006).
- 56.
INFOLEG (1999).
- 57.
INFOLEG (1995).
- 58.
INFOLEG (2002b).
- 59.
INFOLEG (2009).
- 60.
Rumi (2018).
- 61.
Whelan and Lema (2017).
- 62.
- 63.
- 64.
Whelan and Lema (2017).
- 65.
Lusser et al. (2011).
- 66.
SAG (2017).
- 67.
CTNBIO (2018).
- 68.
- 69.
ICA (2018).
References
AOSCA (2018) Seed certification. https://www.aosca.org/programs-and-services/seed-certification/. Accessed 02 May 2018
ASA (2018) Cultivos Bt y manejo de resistencia de insectos. http://www.asa.org.ar/material-de-interes/biotecnologia/. Accessed 02 May 2018
Barfoot P, Brookes G (2014) Global socio-economic and environmental impacts 1996–2014. GM Crops & Food. http://www.pgeconomics.co.uk/pdf/2016globalimpactstudymay2016.pdf. Accessed 02 May 2018
Berto G (2018) Defienden los alimentos genéticamente modificados. Congreso de Bromatología analizó el tema. Afirman que los controles son los adecuados. https://www.rionegro.com.ar/sociedad/defienden-los-alimentos-geneticamente-modificados-AAHRN20071123102002. Accessed 02 May 2018
Biz P (2017) Biotecnología rosarina desarrollará una super soja resistente a herbicidas. https://puntobiz.com.ar/noticias/val/113299/val_s/129/biotecnologica-rosarina-desarrollara-una-super-soja-resistente-a-herbicidas.html. Accessed 02 May 2018
Burachik M (2012) Regulation of GM crops in Argentina. GM Crops Food 3(1):48–51
Burachik M, Traynor PL (2002) Analysis of a national biosafety system: regulatory policies and procedures in Argentina. ISNAR, The Hague. (No. 660.6/B945)
Bustamante E (2018) La Argentina tiene un plan para la biotecnología. CLARIN Rural. https://www.clarin.com/rural/argentina-plan-biotecnologia_0_HkIhhaOBf.html. Accessed 07 Feb 2018
Cascardo R, Gianni C, Piana JA (1998) Variedades vegetales en Argentina: El comercio de semillas y el derecho de obtentor (No. D50/39). Latín Gráfica, Buenos Aires
CBD (2000) Text of the Cartagena protocol on biosafety. http://bch.cbd.int/protocol/text/. Accessed 02 May 2018
CBD (2018a) Parties to the Cartagena protocol and its supplementary protocol on liability and redress. https://bch.cbd.int/protocol/parties/. Accessed 3 Mar 2018
CBD (2018b) COP-MOP decisions. https://www.cbd.int/decisions/mop. Accessed 02 May 2018
Co-Extra (2005) Comparative review of coexistence and traceability systems in selected countries of Europe, North and South America. Deliverable D7.27 of the GM and non-GM supply chains: their CO-E**stence and TRAceability. http://www.coextra.eu/deliverables/deliverable1348.pdf. Accessed 02 May 2018
COMTRADE (2018) UN Comtrade Database 2014-2017. https://comtrade.un.org/. Accessed 02 May 2018
CTNBIO (2018) Resolução Normativa N° 16, de 15 de janeiro de 2018. http://ctnbio.mcti.gov.br/inicio/-/asset_publisher/58KNi0CuF68J/content/resolucao-normativa-n%C2%BA-16-de-15-de-janeiro-de-2018. Accessed 02 May 2018
EFSA (2012) Scientific opinion ad-dressing the safety assessment of plants developed using Zinc Finger Nuclease 3 and other Site-Directed Nucle-ases with similar function. http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2012.2943/epdf. Accessed 02 May 2018
FAO (2000) Codex Alimentarius Commission. Comments submitted by Argentine Governments regarding FOODS DERIVED FROM BIOTECHNOLOGY, page 4 comments on GMO Labeling. http://www.fao.org/tempref/codex/Meetings/CCFBT/ccfbt1/bt0041ce.pdf. Accessed 02 May 2018
FAO (2011) Compilation of Codex texts relevant to the labelling of foods derived from modern biotechnology. http://www.fao.org/fao-who-codexalimentarius/thematic-areas/biotechnology/en/. Accessed 02 May 2018
FAO (2014a) La FAO y Argentina refuerzan la seguridad en biotecnología. http://www.fao.org/director-general/newsroom/news/detail/es/c/264211/. Accessed 02 May 2018
FAO (2014b) Low levels of genetically modified crops in International Food and Feed Trade: FAO International Survey and Economic Analysis. www.fao.org/docrep/019/i3734e/i3734e.pdf. Accessed 02 May 2018
Gepts P, Hancock J (2006) The future of plant breeding. Crop Sci 46(4):1630–1634
Ghezan G, Tapia C (2006) Technical visit report - experiences of traceability: Non-GM Maize in Argentina’, INTA. CO-ExTra Project
Ghezan G, Iriarte L, Tapia C, D’Annunzio J (2006) Argentine non-GMO Soybean Chain, INTA
H. Cámara de Diputados de la Nación (2010) Proyecto de Ley. “Etiquetado de alimentos que sean o contengan Organismos Genéticamente Modificados o sus derivados”. http://www1.hcdn.gov.ar/proyxml/expediente.asp?fundamentos=si&numexp=8307-D-2010. Accessed 02 May 2018
H. Cámara de Diputados de la Nación (2013) Proyecto de Ley. Informacion al consumidor sobre etiquetado de alimentos y bebidas transgenicos o que contengan Organismos Geneticamente Modificados. http://www1.hcdn.gov.ar/proyxml/expediente.asp?fundamentos=si&numexp=2324-D-2013. Accessed 02 May 2018
ICA (2018) SPS Notification G/SPS/N/COL/282. https://docs.wto.org/dol2fe/Pages/FE_Search/FE_S_S009-DP.aspx?language=E&CatalogueIdList=243401&CurrentCatalogueIdIndex=0&FullTextHash=1&HasEnglishRecord=True&HasFrenchRecord=True&HasSpanishRecord=True. Accessed 02 May 2018
INDEC (2018) Database of the National Institute of Statistics and Surveys. https://www.indec.gob.ar/. Accessed 02 May 2018
INFOLEG (1973) Ley de Semillas y creaciones Fitogenéticas. http://servicios.infoleg.gob.ar/infolegInternet/anexos/30000-34999/34822/texact.htm. Accessed 02 May 2018
INFOLEG (1992) Ley de Ministros 22.520. http://servicios.infoleg.gob.ar/infolegInternet/anexos/45000-49999/48853/texact.htm. Accessed 02 May 2018
INFOLEG (1995) Constitucion de la Nacion Argentina. Ley N° 24.430. Publicación del texto oficial de la Constitución Nacional (sancionada en 1853 con las reformas de los años 1860, 1866, 1898, 1957 y 1994). http://servicios.infoleg.gob.ar/infolegInternet/anexos/0-4999/804/norma.htm. Accessed 02 May 2018
INFOLEG (1999) Produccion Ecologica, Biologica u Organica. Ley 25.127. http://servicios.infoleg.gob.ar/infolegInternet/anexos/55000-59999/59885/norma.htm. Accessed 02 May 2018
INFOLEG (2002a) Resolución 412/2002. http://servicios.infoleg.gob.ar/infolegInternet/anexos/70000-74999/74376/norma.htm. Accessed 02 May 2018
INFOLEG (2002b) Política Ambiental Nacional Ley 25.675. http://www.infoleg.gob.ar/infolegInternet/anexos/75000-79999/79980/norma.htm. Accessed 02 May 2018
INFOLEG (2004) Organismos Vegetales Geneticamente Modificados. Resolución 46/2004. http://servicios.infoleg.gob.ar/infolegInternet/anexos/90000-94999/92241/norma.htm. Accessed 02 May 2018
INFOLEG (2007) Ley de Promoción del Desarrollo y Producción de la Biotecnología Moderna, Ley 20270. http://www.infoleg.gob.ar/infolegInternet/anexos/130000-134999/130522/norma.htm. Accessed 02 May 2018
INFOLEG (2009) CODIGO PENAL Ley 26.524 Modificación. http://servicios.infoleg.gob.ar/infolegInternet/anexos/155000-159999/159680/norma.htm. Accessed 02 May 2018
INFOLEG (2011a) Resolución 763/2011. http://servicios.infoleg.gob.ar/infolegInternet/anexos/185000-189999/185806/norma.htm. Accessed 02 May 2018
INFOLEG (2011b) Subsidiary regulations to Resolución 763/2011. http://servicios.infoleg.gob.ar/infolegInternet/verVinculos.do?modo=2&id=185806. Accessed 02 May 2018
INFOLEG (2011c) Resolución 701/2011. http://servicios.infoleg.gob.ar/infolegInternet/anexos/185000-189999/189067/norma.htm. Accessed 02 May 2018
INFOLEG (2011d) Resolución 510/2011. http://servicios.infoleg.gob.ar/infolegInternet/anexos/185000-189999/185853/norma.htm. Accessed 02 May 2018
INFOLEG (2012a) Resolución 241/2012. http://servicios.infoleg.gob.ar/infolegInternet/anexos/195000-199999/198153/norma.htm. Accessed 02 May 2018
INFOLEG (2012b) Resolución 49/2012. http://servicios.infoleg.gob.ar/infolegInternet/anexos/190000-194999/194394/norma.htm. Accessed 02 May 2018
INFOLEG (2013a) Resolución 17/2013. http://servicios.infoleg.gob.ar/infolegInternet/anexos/220000-224999/224874/norma.htm. Accessed 02 May 2018
INFOLEG (2013b) Resolución N° 498/2013. http://servicios.infoleg.gob.ar/infolegInternet/anexos/220000-224999/224698/norma.htm. Accessed 02 May 2018
INFOLEG (2015) Sanidad de los Animales y Vegetales Ley 27233. http://servicios.infoleg.gob.ar/infolegInternet/anexos/255000-259999/257451/norma.htm. Accessed 02 May 2018
INFOLEG (2017a) Resolución 79-E/2017. http://servicios.infoleg.gob.ar/infolegInternet/anexos/285000-289999/287337/norma.htm. Accessed 02 May 2018
INFOLEG (2017b) Resolución Conjunta 9-E/2017. http://servicios.infoleg.gob.ar/infolegInternet/anexos/280000-284999/280580/norma.htm. Accessed 02 May 2018
INFOLEG (2018) Resolución 26/2018. http://servicios.infoleg.gob.ar/infolegInternet/anexos/310000-314999/310441/norma.htm. Accessed 02 May 2018
Ingrassia V (2017) Avance científico mundial: investigadores argentinos lograron generar clones equinos con genomas mejorados. https://www.infobae.com/salud/ciencia/2017/12/04/avance-cientifico-mundial-investigadores-argentinos-logran-generar-clones-equinos-con-genomas-mejorados/. Accessed 02 May 2018
ISAAA (2016) International service for the acquisition of agri-biotech applications. Ithaca, NY. Global Status of Commercialized Biotech/GM Crops: 2016. ISAAA Brief No. 52. ISAAA. https://www.isaaa.org/resources/publications/briefs/52/download/isaaa-brief-52-2016.pdf. Accessed 02 May 2018
ISF (2018) Technological advances drive innovation in plant breeding to create new varieties. http://www.worldseed.org/our-work/plant-breeding/plant-breeding-innovation/. Accessed 02 May 2018
Ishii T, Araki M (2017) A future scenario of the global regulatory landscape regarding genome-edited crops. GM Crops Food 8(1):44–56
Longoni M (2017) La “edición genética”, una nueva técnica que alborota a la industria semillera. Infobae. http://bichosdecampo.com/la-edicion-genetica-una-nueva-tecnica-que-alborota-a-la-industria-semillera/. Accessed 02 May 2018
Lusser MP, Parisi C, Plan D, Rodriguez Cerezo E (2011) New plant breeding techniques – state-of-the-art and prospects for commercial development. http://ftp.jrc.es/EURdoc/JRC63971.pdf. Accessed 02 May 2018
MINAGRO (2018) List of GM events approved in Argentina. https://www.agroindustria.gob.ar/sitio/areas/biotecnologia/ogm/. Accessed 02 May 2018
MINCYT (2015) Encuesta Exploratoria Sobre Percepción Pública de la Biotecnología Alimentaria en la Argentina. http://indicadorescti.mincyt.gob.ar/documentos/Informe_Percepcion_Biotecnologia_Alimentos.pdf. Accessed 02 May 2018
OECD (2015) Workshop on environmental risk assessment of products derived from novel plant breeding techniques. https://one.oecd.org/document/ENV/JM/MONO(2016)5/en/pdf. Accessed 02 May 2018
OECD (2018) OECD seed schemes: rules and regulations. http://www.oecd.org/tad/code/oecdseedschemesrulesandregulations.htm. Accessed 02 May 2018
Penna JA, Lema MA (2003) Adoption of herbicide tolerant soybeans in Argentina: an economic analysis in the economic and environmental impacts of Agbiotech. Springer, Berlin, pp 203–221
Pensel N, Gargiulo G, Barrios M (2007) Traceability, coexistence systems and practices in Argentina. INTA
Phillips PWB, Smyth S (2002) Product differentiation alternatives: identity preservation, segregation, and traceability. AgBioforum 5(2):30–42
Preciado J (2015) La biotecnología después de la transgenia. Infocampo. http://www.infocampo.com.ar/la-biotecnologia-despues-de-la-transgenia/. Accessed 02 May 2018
Roman V (2017) Edición genética en Argentina: cómo se usa para beneficiar a pacientes, mascotas y productores agropecuarios. Infobae. https://www.infobae.com/salud/ciencia/2017/08/17/edicion-genetica-en-argentina-como-se-usa-para-beneficiar-a-pacientes-mascotas-y-productores-agropecuarios/. Accessed 02 May 2018
Rosada C (2018) Ley de desarrollo y promoción de biotecnología. https://www.casarosada.gob.ar/informacion/eventos-destacados-presi/41676-se-reglamento-la-ley-de-desarrollo-y-promocion-de-biotecnologia. Accessed 18 Jan 2018
Rumi MJ (2018) La biotecnología argentina apunta al exterior. La Nación. https://www.lanacion.com.ar/2101488-la-biotecnologia-argentina-apunta-al-exterior. Accessed 02 May 2018
SAG (2017) Aplicabilidad de Resolución N° 1.523/2001 en material de propagación desarrollado por nuevas técnicas de fitomejoramiento. http://www.sag.gob.cl/ambitos-de-accion/aplicabilidad-de-resolucion-ndeg-15232001-en-material-de-propagacion-desarrollado-por-nuevas-tecnicas-de-fitomejoramiento. Accessed 02 May 2018
Schiavone E, Morón P, Lema M (2013) Normas locales sobre identificación de “alimentos transgénicos” y el “derecho a la información del consumidor” Revista Alimentos Argentinos N° 32. http://www.alimentosargentinos.gob.ar/HomeAlimentos/Publicaciones/revistas/nota.php?id=436. Accessed 02 May 2018
SENASA (2018) Se constató la destrucción de 147 hectáreas de alfalfa transgénica ilegal. http://www.senasa.gob.ar/senasa-comunica/noticias/se-constato-la-destruccion-de-147-hectareas-de-alfalfa-transgenica-ilegal. Accessed 02 May 2018
Sprink T, Metje J, Schiemann J, Hartung F (2016) Plant genome editing in the European Union to be or not to be a GMO. Plant Biotechnol Rep 10(6):345–351
Trigo EJ (2016) Veinte Años de Cultivos Genéticamente Modificados en la Agricultura Argentina. http://argenbio.org/index.php?action=novedades¬e=747. Accessed 02 May 2018
Trigo E, Chudnovsky D, Cap E, López A (2002) Los transgénicos en la agricultura argentina. Una historia con final abierto. Libros Del Zorzal, Buenos Aires
Valor Soja (2017) Incorporaron al aceite de soja alto oleico al Código Alimentario Argentino. https://www.valorsoja.com/2017/10/09/incorporaron-al-aceite-de-soja-alto-oleico-al-codigo-alimentario-argentino-estaria-disponible-en-los-proximos-anos/. Accessed 02 May 2018
Whelan AI, Lema MA (2015) Regulatory framework for gene editing and other new breeding techniques (NBTs) in Argentina. GM Crops Food 6:253–265
Whelan AI, Lema MA (2017) A research program for the socioeconomic impacts of gene editing regulation. GM Crops Food 8(1):74–83
WHO (2018) Laboratory biosafety manual. http://www.who.int/csr/resources/publications/biosafety/WHO_CDS_CSR_LYO_2004_11/en/. Accessed 02 May 2018
WTO (2018) Measures affecting the approval and marketing of biotech products. https://www.wto.org/english/tratop_e/dispu_e/cases_e/ds293_e.htm. Accessed 02 May 2018
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Appendices
Annex 1: First NBT Regulations from LA Countries
2.1.1 Resolution No. 173/2015 (Argentina)
Note: The following text is offered for illustrative purposes only and it is not an official translation (Spanish is the official language of the Argentine Republic).
In Considering:
Resolution No. 763 dated August 17, 2011 of the Ministry of Agriculture, Livestock and Fisheries (MAGYP) sets forth the guidelines for the activities involving Genetically Modified Organisms (GMO) in the Republic of Argentina.
Pursuant to article 3.A of the Resolution No. 763/11, risk assessment, design of biosafety measures and risk management during each stage of GMO assessment hereof shall be conducted by the National Advisory Commission on Agricultural Biotechnology (CONABIA), which Executive Secretariat is held by the Biotechnology Directorate of the National Directorate of Processes and Technologies of the Undersecretariat of Added Value and New Technologies under the Secretariat of Agriculture, Livestock and Fisheries (SAGYP) under the MAGYP.
Article 3 of Resolution (SAGYP) No. 437 dated August 06, 2012 sets forth as actions pertaining to CONABIA, among others, to advise the Secretary of Agriculture, Livestock and Fisheries on “risk assessment, design of biosafety measures and risk management in the various stages of assessment, authorization and release into the agro-ecosystem of genetically modified organisms” and “every issue to be submitted to its scientific evaluation”.
Resolution (SAGYP) No. 701 dated October 27, 2011 sets forth the requirements and proceedings that must be met by biosafety assessments for the release of GM-plants into the agro-ecosystem.
Resolution No. 701/11 defines GM-plant as a plant organism bearing a combination of genetic material obtained through the application of modern biotechnology.
Such regulation defines event as “the combined and stable insertion into the plant genome of one or more genes or DNA sequences that are part of a defined genetic construct”.
The development of agricultural biotechnology is a key tool for the addition of value in the agribusiness value chain in the Argentine Republic.
In the Argentina Republic, as in the rest of the world, major advances are being produced in the development of new breeding techniques in plants (NPBT).
The characteristics of the crops derived from these techniques are of such heterogeneity that demand a prior scientific assessment in order to determine whether any such crop falls under the rules and regulations applicable to GM-plants or, on the contrary, are not subject to such regulations.
That this decision does not alter the regulatory framework applicable to GMO but rather sets forth proceedings to determine the cases in which a crop obtained by NBT that use modern biotechnology to generate genetic modifications are subject to GMO rules and regulations.
CONABIA, after an extended debate in several meetings during 2013 and 2014 has rendered its agreement to this regulation during its ninth meeting of the year 2014, which took place on November 25, 2014.
The General Directorate of Legal Affairs of the Ministry of Agriculture, Livestock and Fisheries has expressed its legal opinion.
The Secretary of Agriculture, Livestock and Fishery is competent to issue this resolution pursuant to Decree No. 357 dated February 21, 2002 as amended.
Therefore, the Secretary of Agriculture, Livestock and Fisheries Hereby Orders as Follows:
Article 1
The procedure to determine in which cases a crop obtained by new breeding plant techniques (NBPT) using modern biotechnology, does not fall under GMO rules and regulations pursuant to Resolution (MAGYP) No. 763 dated August 17, 2011 and its complementary regulations, is hereby enacted.
Article 2
In regards to the situations mentioned in Article 1, the interested party shall submit its case for the assessment of CONABIA through a Previous Consultation Stage (“ICP”) pursuant to Resolution No. 701/11. During the ICP the Applicant shall submit data on the breeding methodology used to obtain and select the crop, on the new trait or characteristic introduced, and on evidence of the genetic changes present in the final product. Within the framework of the ICP, the applicant shall request CONABIA to establish whether the result of the breeding process is a novel combination of genetic material.
A genetic change shall be regarded as a novel combination of genetic material when the assessment establishes the occurrence of a stable and joint insertion in the plant genome of one or more genes or DNA sequences being part of a defined genetic construct.
Article 3
Any GM-plant offspring shall be presumed also a GM-plant until scientific information proves otherwise. Therefore, in addition to the provisions contained in Article 2 herein, applicants shall inform if any transformation event was used during the breeding process, even when it is no longer present in the crop to be introduced into the agro-ecosystem, and include evidence of its absence.
Article 4
The Biotechnology Directorate will conduct a preliminary assessment on the data provided by applicants in a period not exceeding 60 calendar days, and proceed to list the matter for debate in the following CONABIA meeting. On the basis of the information filed during the ICP, CONABIA will establish whether a novel combination of genetic material has been created. Also, if appropriate, CONABIA will determine if there exists enough scientific evidence to support the absence of the event(s) used transiently during the crop breeding process. Both the Biotechnology Directorate and CONABIA may request the Applicants to file additional data and information in order to complete their assessments.
Article 5
Upon CONABIA finding that a novel combination of genetic material has not been created and, if applicable, that no unauthorized events subsist in the crop, the SAGYP, through the Biotechnology Directorate shall notify the Applicant that the product does not fall under the scope of Resolution No. 763/11 and its complementary regulations.
Notwithstanding the aforementioned, CONABIA may also recommend the Secretary of Agriculture, Livestock and Fisheries, the adoption of follow-up measures on a case-by-case basis, taking into account the crop features and/or novelty, based on scientific and technical grounds.
Article 6
Applicants must be previously registered under the National Registry of Genetically Modified Plants Organisms Operators (RNOOVGM) created by Resolution (ex-SAGPYA) No. 46 dated January 7, 2004 before filing for the ICP. Otherwise, applicants will be subject to register with the Biotechnology Directorate in order to prove their legal standing. If the product is considered a GM-Plant, applicants must register under the RNOOVGM before continue filing their first application for GM-Plant environmental release.
Article 7
Alternatively, applicants may file for a preliminary inquiry aiming at anticipating whether a hypothetical product from projects still in the design stage would fall under the scope of Resolution No. 763/11 and its complementary regulations. In these cases, no registration under the RNOOVGM or equivalent documentation shall be required and CONABIA shall perform a preliminary assessment and provide an indicative answer that the Biotechnology Directorate will notify to applicants. If such new crops are obtained later, they shall be subjected to the provisions hereinabove in order to establish whether they have the features anticipated in the preliminary inquiry.
Article 8
This resolution shall come into effect the day after its publication in the Official Gazette.
Article 9
Be it communicated, published, given to the National Directorate of the Official Registry and filed.-.Sgd.: G DELGADO. Secretary of Agriculture, Livestock and Fisheries.
2.1.2 Resolution No. 16/2018 (Brazil)
Note: The following text is offered for illustrative purposes only and it is not an official translation (Portuguese is the official language of the Federative Republic of Brazil).
Ministry of Science, Technology, Innovations and Communications
National Biosafety Technical Committee
The National Technical Commission on Biosafety—CTNBio, in the use of its legal and regulatory attributions and in compliance with the provisions contained in items XV and XVI of art. 14 of Law 11,105 of March 24, 2005;
Considering
The need to consider the Innovative Precision Improvement Techniques (TIMP), from the English Precision Breeding Innovation (PBI) which also encompasses the so-called New Breeding Technologies-NBTs, in the light of the precepts provided in the Law No. 11,105 of March 24, 2005;
Considering that Law No. 11,105 of 2005 defines recombinant DNA/RNA molecules, genetic engineering and genetically modified organisms—GMOs in items III, IV and V of its art. 3, respectively;
Considering that TIMP encompass a set of new methodologies and approaches that differ from the genetic engineering strategy by transgene, as it results in the absence of recombinant DNA/RNA in the final product;
Considering that TIMP can introduce innovative uses of molecular biology tools, which can result in:
-
1.
The precise editing of genomes, by induction of specific mutations, generating or modifying wild and/or mutated alleles without transgene insertion (s);
-
2.
Genetic transformation and/or control of gene expression (activation/inactivation);
-
3.
Epigenetic regulation of the expression of genes by natural mechanisms without genetic modification of the individual;
-
4.
Genetic transformation and/or control of gene expression with genes from sexually compatible species;
-
5.
Temporary and non-inheritable genetic transformation of cells and tissues;
-
6.
Permanent or non-permanent host infection of genetically modified viral elements;
-
7.
The creation of alleles with autonomous inheritance and potential of recombination with the possibility of altering a whole population (gene drive); and
-
8.
The construction of heterologous genes or new copies of homologous genes
Resolves
Article 1
The technologies described in Annex 2 which are part of this Normative Resolution, may originate a product not considered as a Genetically Modified Organism (GMO) and its derivatives, as defined by Law No. 11,105 of March 24, 2005, are considered examples of Innovative Precision Improvement Techniques (TIMP), but not limited to them.
-
§ 1—The product referred to in the section of this article is defined as the offspring, lineage or end product of a process that uses Innovative Precision Improvement Techniques in one of its phases of development.
-
§ 2—The cases to be classified are not limited to the technologies described in Annex 2, considering that different technologies are rapidly and continuously advancing and may provide new products, to which the provisions of this Normative Resolution will also apply.
-
§ 3—The products referred to in the main paragraph of this article imply at least one of the following characteristics:
-
I
product with proven absence of recombinant DNA/RNA, obtained by a technique employing GMOs as a parent;
-
II
product obtained by a technique using DNA/RNA that will not multiply in living cells;
-
III
product obtained by a technique that introduces targeted site mutations, causing gain or loss of gene function, with the proven absence of recombinant DNA/RNA in the product;
-
IV
a product obtained by a technique where there is a temporary or permanent expression of recombinant DNA/RNA molecules, without the presence or introgression of these molecules in the product; and
-
V
a product where techniques employing DNA/RNA molecules are used which, whether absorbed or not systemically, do not cause permanent modification of the genome.
In the case of a product obtained from a GMO with favorable opinion from CTNBio for commercial release, the conditions described will apply only to the characteristic introduced by TIMP.
Article 2
In order to determine whether the product obtained by TIMP will be considered as a GMO and its derivatives, pursuant to article 3 of Law 11,105 of 2005, the applicant must submit a consultation to CTNBio.
-
§ 1—The consultation shall be accompanied by the information contained in Annex 3 of this Normative Resolution.
-
§ 2—Once the consultation is registered at CTNBio, its view will be published in the Official Journal of the Union and distributed to one of the members, titular or alternate, for reporting and drawing up a final opinion.
-
§ 3—The final opinion of the member shall be based on an analysis, on a case-by-case basis, of proof of compliance with at least one of the conditions described in § 3° of article 1 of this Normative Resolution.
-
§ 4—For the products and technologies obtained using the techniques exemplified in Annex 2, CTNBio’s decision shall observe compliance with one or more of the conditions described in § 3 of article 1 of this Normative Resolution and will be conclusive regarding the application of the definitions of arts. 3 and 4 of Law 11,105 of 2005.
Article 3
The final opinion referred to in paragraphs 2nd of art. 2nd of this Normative Resolution shall be submitted to at least one of the Standing Sectoral Subcommittees, in agreement with the parental organism and the proposed use of the technique submitted for consultation and, after its approval, shall be referred to the CTNBio plenary for deliberation.
The Subcommittees shall within 90 days analyze and elaborate the opinions, and this term may be extended for the same period by decision at the CTNBio plenary.
Article 4
CTNBio may, as a result of the consultation and with due scientific justifications, request additional information or studies.
Article 5
Any situations not foreseen herein will be assessed and decided, case by case, by CTNBio.
Article 6
This Normative Resolution enters into force on the date of its publication.
EDIVALDO DOMINGUES VELINI
President of the Commission
Annex 2
Examples of “Innovative Precision Improvement Techniques (TIMP)”.
-
1.
Technique: Induced Early Flowering.
-
1.1
Summary of the Technique: Silencing and/or overexpression of genes related to flowering by insertion of the genetic modification into the genome and subsequent segregation or through temporary expression by viral vector.
-
2.
Technique: Technology for Seed Production.
-
2.1
Summary of the Technique: Insertion of the genetic modification for restoration of fertility in naturally male-sterile lines in order to multiply these lines maintaining the male-sterility condition, without, however, transmitting the genetic modification to the offspring.
-
3.
Technique: Reverse Breeding improvement.
-
3.1
Summary of the Technique: Inhibition of meiotic recombination in selected heterozygous plants for the characteristic of interest in order to produce homozygous parental lines.
-
4.
Technique: Methylation of RNA-Dependent DNA.(RNA-directed DNA methylation)
-
4.1
Summary of the Technique: Methylation directed by interfering RNAs (“RNAi”) in promoter regions homologous to RNAi with the objective of inhibiting the transcription of the target gene in living beings.
-
5.
Technique: Site Directed Mutagenesis.
-
5.1
Summary of the Technique: Protein or riboprotein complexes capable of causing site-directed mutagenesis in microorganisms, plants, animals and human cells.
-
6.
Technique: Oligonucleotide Directed Mutagenesis.
-
6.1
Summary of the Technique: Introduction into the cell of an oligonucleotide synthesized complementary to the target sequence, containing one or a few nucleotide changes, which may cause substitution, insertion or deletion in the target sequence through the cell repair mechanism (microorganisms, plants, animals and human cells).
-
7.
Technique: Agroinfiltration/Agroinfection.
-
7.1
Summary of the Technique: Leaves (or other somatic tissue) infiltrated with Agrobacterium sp. or gene constructs containing the gene of interest to obtain temporary expression at high levels located in the infiltrated area or with viral vector for systemic expression, without the modification being transmitted to subsequent generations.
-
8.
Technique: Topical application of RNAi/ systemic use.
-
8.1
Summary of the Technique: Use of double stranded RNA (“dsRNA”) sequence homologous to the target gene (s) for specific silencing of such gene (s). The engineered dsRNA molecules can be introduced/absorbed by the cell from the environment.
-
9.
Technique: Viral Vector.
-
9.1
Summary of the Technique: Inoculation of living organisms with recombinant virus (DNA or RNA) expressing the genetic modification and amplification of the gene of interest through the mechanisms of viral replication, without modification of the host genome.
Annex 3
-
1.
Regarding to the original organism (parental organisms), indicate:
-
1.1.
the identification of the genetic technology, purpose and intended use of the resulting organism and its derivatives;
-
1.2.
the taxonomic classification, from family, to the most detailed level of the organism to be released, including, where appropriate, subspecies, cultivar, pathovar, strain and serotype;
-
1.3.
the risk classification of the genetically modified organism in accordance with Normative Resolution No. 2 of November 27, 2006 4. the gene(s) and/or genetic element(s), body(ies) of origin and their specific functions, where applicable;
-
1.4.
the genetic strategy (s) used to produce the desired modification (s); the genetic map (s) of the building (s) used in the process indicating, with all genetic elements present;
-
1.5.
Molecular characterization of the result of manipulation in the recipient organism (parent and end product), where applicable, providing information related to: (1) number of manipulated copies (e.g. number of genomic sequences, number of alleles, etc.); (2) location in the genome of the manipulated region, where possible; (3) identify the presence of unintentional genetic modifications (off-target), when applicable.
-
1.6.
the product of expression of the manipulated genomic region (s), described in detail, where applicable.
-
2.
With regard to the product (offspring, lineage or final product) inform:
-
2.1.
proof of the absence of recombinant DNA/RNA molecules, through the use of molecular methods.
-
2.2.
whether the product containing DNA/RNA molecules for topical/systemic use has the recombinant ability to enter into target species and/or non-target species.
-
2.3.
whether the product covered by the application is commercially approved in other countries.
-
2.4.
If the product uses the gene drive principle that may allow the phenotypic change conferred to have the potential to spread throughout the recipient organism population, explain the care to monitor the organism using at least two strategies.
-
2.5.
how the possibility of possible unintentional (off-target) effects of the technology that may be present on the product has been assessed.
2.1.1 Guidance on the Applicability de Resolution no. 1.523/2001 (Chile)
Note: The following text is offered for illustrative purposes only and it is not an official translation (Spanish is the official language of the Republic of Chile).
Applicability of Resolution No. 1.523/2001 on propagation material developed by new plant breeding techniques.
Individuals, natural or legal who want to internalize and introduce to the environment live modified plant propagation organisms, must strictly comply with the provisions of Exempt Resolution No. 1523 of 2001, of the Agricultural and Livestock Service (SAG). [1]
Considering that scientific progress has allowed the development of a new generation of biotechnological techniques of plant genetic improvement other than transgenics, the Agricultural and Livestock Service has considered it necessary to solve case by case if the propagative material developed by any of these techniques is within or outside the scope of Resolution No. 1523 of 2001.
In this context the SAG, based on the information presented by the interested party that intends to introduce to the national environment, a propagation material developed by any of these techniques, whether imported or national, will be pronounced by resolution, with respect to if said material is within or beyond the scope of Resolution No. 1523 of 2001. To this end, the SAG will evaluate the background information presented on the technique and verify whether the propagation material in question has a novel combination of genetic material.
For these purposes, a novel combination of genetic material will be understood as a stable insertion of one or more genes or DNA sequences that encode proteins, interfering RNA, double-stranded RNA, signaling peptides or regulatory sequences.
This procedure allows to obtain an official statement from the SAG, through a Resolution, that will indicate whether the propagation material developed by any of the new biotechnological techniques of plant breeding, which is intended to be introduced into the national environment, is within or outside the scope of Resolution No. 1523 of 2001, which for this purpose means that the material is considered or not LMOs, respectively.
In the event that the SAG determines that the propagation material submitted for the Service’s consideration is outside the scope of Resolution No. 1523 of 2001, the interested party may carry out activities for the purposes of agricultural production and use, without restrictions and therefore both without having to comply with the biosecurity measures established by the SAG, in accordance with the aforementioned Resolution in force.
The pronouncement will have an indefinite validity or until the Service determines otherwise based on new scientific background.
To whom is addressed:
Natural or legal persons, research centers or universities that intend to introduce to the national environment a propagative material produced from a new plant breeding technique different from transgenics.
Documentation to present
The presentation made by the interested party in introducing propagation material produced from new plant breeding techniques other than transgenics into the environment, should be addressed to the Chief of the Division of Agricultural and Forestry Protection, consulting as to whether the material is outside or within the scope of Resolution No. 1523 of 2001, in consideration of the information that is presented and exists about said material and its development process.
For these purposes, the application is submitted through the form established by the SAG, containing and accompanying the information in this required, at least [2]:
All information must be presented in Spanish and attach all reference articles, analytical results and documentation from official agencies that support its content.
The sole intention of introducing another type of propagation material, imported or national, to the environment in the country, in different stages of development, will require the presentation of a new application.
All the information delivered by the user will be protected according to the current regulations.
Response time: 20 business days, from the receipt of the request by the Division of Agricultural and Forestry Protection of the SAG.
Cost of the procedure: The evaluation of the background will be governed by the Supreme Decree No. 142 of 1990 of the Ministry of Agriculture.
[1] Breaches of current regulations on the subject will be sanctioned according to the Law of Agricultural Protection (Decree Law No. 3,557) and the procedure established in Law No. 18.755 Organic Agriculture and Livestock Service.
[2] The veracity of the information provided will be the sole responsibility of the applicant and in no case exempts it from compliance with other regulations that apply to the same material.
Form
The undersigned that is identified below, comes to present to you for processing an official statement on whether the propagation material developed by a new biotechnological techniques of plant breeding, which is intended for introduction into the national environment, is within or outside the scope of Resolution No. 1523 of 2001, which establishes standards for the confinement and introduction into the environment of living modified plant propagation organisms.
Section II: Technical Information Presented (∗∗)
Section I: Identification of the Applicant
-
1.
Applicant’s background:
-
Name or company name:
-
Genre:
-
Natural person or Legal person
-
Nationality (only natural person case):
-
Home address:
-
Commune: Region: Country:
-
Email: Phone number:
-
2.
Background of the legal representative (only in case of legal entity):
-
Name of the legal representative (∗):
-
Nationality:
-
Gender: F M
-
Address in Chile:
-
Commune: Region:
-
3.
Background of the technical counterpart before the SAG:
-
Name of the technical counterpart before the SAG:
-
E-mail technical counterpart:
-
Phone number:
(∗) Accompany document stating the power conferred in accordance with Law No. 19,880.
Section II: Technical Information Presented (∗∗)
-
1.
Individualization of the propagation material to be introduced into the environment:
-
a.
Species.
-
b.
Variety/Line.
-
c.
Description of obtained phenotype.
-
d.
Company or institution that developed the material.
-
2.
Regarding the biotechnological process used:
-
2.1
Background of the biotechnological technique used, indicating the modified DNA sequences.
-
2.2
Include genetic scheme detailing the lines that will be introduced in Chile and the techniques used to rule out the insertion of genetic sequences that encode proteins, RNA interference, double-stranded RNA, signaling peptides or regulatory sequences.
-
3.
Indicate if the propagation material has been authorized by the official agency of a country. If this is the case, you must indicate the type of authorization referring exclusively to the material that is requested to be entered into the national environment, providing all the written information that you have.
(∗∗) The information must be presented in Spanish and attach all reference articles, analytical results and documentation from official agencies that support its content.
Along with the above, I declare under oath to be aware of the following:
-
1.
The veracity of the information provided will be the sole responsibility of the applicant and in no case exempts it from compliance with other regulations that apply to the material.
-
2.
The sole intention of introducing another type of propagation material, imported or national, to the environment in the country, at different stages of development, will require the submission of a new application.
Signature of the applicant.
2.1.2 Draft Resolution (Colombia)
Note: The following text is the translation provided by the Colombian Government to the WTO on its notification G/SPS/N/COL/282 presented to the Committee on Sanitary and Phytosanitary Measures (Spanish is the official language of the Colombia Republic).
Draft Resolution setting out the applicable procedure for crops where any stages over the plant-breeding process incorporate innovative phyto-improvement techniques through modern biotechnology and the final product does not contain any foreign genetic material.
Resolution No. (to be assigned)
The General Manager of the Colombian Agricultural & Farming Institute (ICA)
In use of his legal powers and particularly as conferred by Article 65 under Act 101 of 1993, Article 4, Decree 3761 of 2009 and Article 2.13.1.6.1, Decree 1071 of 2015 and
In Consideration That:
In accordance with Article 65, Act 101 of 1993 “General Agricultural, Farming & Fishing Development Act”, it is the responsibility of the Ministry of Agriculture, through the Colombian Agricultural & Farming Institute—ICA, to develop policies and plans intended for protection of nationwide agricultural and farming health, production and productivity; therefore, it shall be responsible for undertaking agricultural and farming health actions and exerting technical control over imports, exports, manufacturing, commercialization and use of agricultural and farming feedstock intended for protecting domestic agricultural and farming production and minimizing the risks for foods and the environment arising from use thereof and facilitating access of domestic products to the international market.
By means of Act 740 of 2002 Colombia ratified the Cartagena Protocol on Biosafety to the Convention on Biological Diversity, the purpose of which, in accordance with the precaution approach, is to contribute to guaranteeing an appropriate level of protection within the realm of safe transfer, manipulation and use of Living Modified Organisms generated by modern Biotechnology that may have adverse effects on preservation and sustainable use of biological diversity and specifically focusing on cross-border movements.
Over development of such provisions, the Colombian National Government issued Decree 4525 on 6th December 2005 “Regulating Act 740 of 2002” and set out that the Ministry of Agriculture & Rural Development, by means of the Colombian Agricultural & Farming Institute—ICA, shall be competent for authorizing the activities described in Article 2 therein when dealing with Living Modified Organisms—LMO exclusively for agricultural, farming, fishing, commercial forest plantation and agroindustrial use, as may have adverse effects on preservation and sustainable use of biological diversity.
By means of Resolution 0946 of 2006, ICA established the procedure for processing at such bureau applications for Living Modified Organisms—LMO, approving the internal CTNBio LMO by-laws exclusively for agricultural, farming, fishing, commercial forest plantation and agroindustrial purposes issuing other related provisions.
The Colombian Agricultural & Farming Institute—ICA is responsible for exerting technical control over production and commercialization of agricultural and farming feedstock, animal genetic material and seeds for sowing, with the purpose to prevent risks that may affect agricultural and farming health, food safety and domestic agricultural and farming production.
ICA is the agency in charge of granting, suspending or revoking licenses, registrations and permits for operation, commercialization, mobilization, importation or exportation of animals, plants, feedstock, seeds and agricultural and farming products and by-products, as well as imposing any applicable penalties, pursuant to current legal standards.
Dynamic innovation in phyto (vegetable) improvement/plant breeding allows for obtaining heterogeneous products, which makes it necessary to conduct prior technical analysis with the purpose to determine whether the regulation on Living Modified Organisms (LMO) shall be applied thereto.
On the basis of the above, the present Resolution sets forth the procedure that must be applied to crops obtained by means of using phyto-improvement innovation techniques through modern biotechnology where the final product does not contain any foreign genetic material in order to determine if it is LMO or not and consequently decide whether said regulation on Living Modified Organisms (LMO) shall be applied thereto or not.
By virtue of the above,
It Is Hereby Resolved As Follows:
Article One—Purpose: Set out the procedure applicable to crops where any stages over the plant-breeding process incorporate innovative phyto-improvement techniques through modern biotechnology and the final product does not contain any foreign genetic material, for which it shall not be considered as LMO.
Article Two—Scope of Application: The present Resolution shall be applicable to all individuals or companies committed to genetic improvement research, assessing, producing, conditioning, importing, exporting, storing and/or marketing crops that have been materials for sowing.
Article Three—Definitions: For the purposes of this resolution, the following definitions are adopted:
-
3.1
Crop: Generic name used to refer indistinctly to varieties, lines, hybrids and clones that are being used as commercial planting materials.
-
3.2
Phyto-Improvement: This is the art and science of altering or modifying the heredity of plants to obtain genetically improved crops (varieties or hybrids), adapting to specific conditions and resources of the producer, industry and consumers.
-
3.3
Plant Breeding Innovation: This corresponds to scientific progress over the last few years, which has allowed for develo** a new generation of methods/techniques based on refinement of existing modern biotechnology methods designed for increasing phyto-improvement speed, accuracy and efficiency.
-
3.4
Foreign Genetic Material: This refers to exogenous stable and joint insertion in a genome of one (1) or more genes or DNA sequences forming part of a specific genetic construction.
-
3.5
Unintentional (Off-Target) Modifications: These occur when over the phyto-improvement process of a crop innovation techniques through modern biotechnology have been used and the final product—the crop—contains unplanned modifications in its genome.
-
3.6
Living Modified Organism (LMO): Any live organism having a new genetic material combination that has been obtained by means of applying modern biotechnology.
Article Four—Application: Individuals or companies interested in processing an application for having used innovation techniques over phyto-improvement of a crop through modern biotechnology with the final product not containing any foreign genetic material, shall be previously registered with ICA as seed producers or importers or as phyto-improvement research units and shall submit to ICA the respective application meeting the following requirements:
-
4.1
Full name or corporate purpose, address, telephone number, electronic mail address, name and identification of legal representative and proxy, if applicable, and full name or corporate purpose of develo** entity.
-
4.2
For companies, certificate of existence and legal representation issued by the respective Chamber of Commerce with date no older than thirty (30) calendar days before submission of the application; and for individuals, commercial registration, RUT (Single Taxation Registration) or Colombian Citizenship Card.
-
4.3
The application shall indicate the number of the resolution whereby ICA granted registration of the business activity (seed producer or importer or phyto-improvement research unit).
-
4.4
Provide information related to:
-
Crop: Taxonomic species classification, description of phenotype obtained and use.
-
The improvement machinery used for obtaining the desired result, genetic map(s) of construction(s) used over the improvement process, including all present genetic elements, protein sequences and RNA used in the free DNA edition process.
-
Details of any new characteristics or modifications of existing characteristics.
-
Evidence of genetic changes present in the final product—molecular characterization, describing the number of genes, sites, loci or DNA sequences manipulated, location in the genome and, where applicable, identification of any unintentional (off-target) modifications.
-
Analytical evidence showing that the improved crop (final product) does not contain any foreign genetic material.
-
Evidence (by means of DNA sequences) that off-target sites, those that could have predictably been intentionally modified, did not sustain any changes in the improved crop.
Proviso One: The above information shall be submitted in Spanish attaching all reference articles/papers, as well as analytical results.
Proviso Two: Individuals or companies interested in conducting phyto-improvement research shall be previously registered with ICA as research units. In addition, if over the phyto-improvement (plant breeding) process any foreign genetic material was introduced, it shall be reported to ICA irrespective of the final product containing or not any such foreign genetic material. Furthermore, they shall count on authorization from ICA to perform any research activities on a confined basis in meeting the applicable regulations and the established biosafety plan.
Article Five—Processing Applications: Upon submitting an application, within a maximum period of thirty (30) working days counted as from the filing date thereof, ICA shall review the information and documents described in Article Four of the present Resolution, as applicable, and shall require the interested party, where applicable, to clarify any of the information provided or attach any additional documents, for which a maximum period shall be granted up to thirty (30) working days counted as from the date when the communication is received.
Upon expiry of such period, if the interested party has not clarified the information or sent the documents required, it shall be considered that it desists from the application and ICA shall proceed with return thereof complete with the respective supporting documents within the next fifteen (15) working days, without prejudice to the interested party’s right to submit a new application in meeting all the requirements set out in the present Resolution.
Article Six—Response To Application: Once the requirements set out in the above Article have been complied with, ICA shall carry out an assessment of the information received within a period no longer than sixty (60) working days, determining whether the new crop contains any foreign genetic material inserted in its genome due to the use of modern biotechnology techniques.
For a genetic change to be considered as foreign genetic material, it shall be analyzed whether a stable and joint exogenous insertion has been produced in one (1) or more genes or DNA sequences forming part of a specific genetic construction.
Upon determining the above or upon expiry of the period initially indicated, ICA shall inform the applicant in writing if the submitted crop is considered an LMO or not and consequently whether it is or not within the scope of regulation of Living Modified Organisms.
Proviso One: When dealing with a crop obtained by innovative phyto-improvement techniques through modern biotechnology and the final product does not contain any foreign genetic material, for marketing seeds from such crop the applicant shall comply with the provisions of ICA Resolution 3168 of 2015 or any other amending o replacing it or adding thereto, and when dealing with LMO it shall comply with the provisions in Decree 4525 of 2005 and regulatory resolutions thereto and ICA Resolution 3168 or 2015 or any standards amending or replacing it or adding thereto.
Proviso Two: An analysis conducted on one variety/hybrid shall be applicable to another such variety/hybrid of the same species as long as the second variety or both varieties are derived from the same parent as initial source of the new characteristic obtained by means of new phyto-improvement technologies derived from modern biotechnology. The Authority reserves the right to request more information in case it deems so relevant or convenient, on the basis of scientific criteria.
Proviso Three: ICA may request special follow up of any crop analyzed when the characteristics and/or novelty thereof merit so, on the basis of scientific and technical criteria.
Article Seven—Validity: The present Resolution shall govern as from the date of its publication in the Official Journal.
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Whelan, A.I., Lema, M.A. (2019). Regulation of Genome Editing in Plant Biotechnology: Argentina. In: Dederer, HG., Hamburger, D. (eds) Regulation of Genome Editing in Plant Biotechnology. Springer, Cham. https://doi.org/10.1007/978-3-030-17119-3_2
Download citation
DOI: https://doi.org/10.1007/978-3-030-17119-3_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-17118-6
Online ISBN: 978-3-030-17119-3
eBook Packages: Law and CriminologyLaw and Criminology (R0)