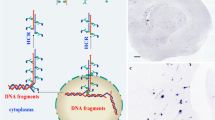
Abstract
The in situ ligation (ISL) methodology detects apoptotic cells by the presence of characteristic DNA double-strand breaks. A labeled double-stranded probe is ligated to the double-strand breaks in situ on tissue sections. Like the popular TUNEL assay, ISL detects cells in apoptosis based on the ongoing destruction of DNA by apoptotic nucleases. In comparison to TUNEL, it is more specific for apoptosis versus other causes of DNA damage, both repairable damage and necrosis. In the decade and a half since its introduction, ISL has been used in several hundred publications. Here we review the development of the method, its current status, and its uses and limitations.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Didenko, V.V., Wang, X., Yang, L., and Hornsby, P.J. (1999) DNA damage and p21WAF1/CIP1/SDI1 in experimental injury of the rat adrenal cortex and trauma-associated damage of the human adrenal cortex. J. Pathol. 189, 119–126.
Gavrieli, Y., Sherman, Y., and Ben-Sasson, S.A. (1992) Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J. Cell Biol. 119, 493–501.
Steigerwald, S.D., Pfeifer, G.P., and Riggs, A.D. (1990) Ligation-mediated PCR improves the sensitivity of methylation analysis by restriction enzymes and detection of specific DNA strand breaks. Nucleic Acids Res. 18, 1435–1439.
Didenko, V.V., and Hornsby, P.J. (1996) Presence of double-strand breaks with single-base 3′ overhangs in cells undergoing apoptosis but not necrosis. J. Cell Biol. 135, 1369–1376.
Wyllie, A.H. (1980) Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature 284, 555–556.
Didenko, V.V., Tunstead, J.R., and Hornsby, P.J. (1998) Biotin-labeled hairpin oligonucleotides. Probes to detect double-strand breaks in DNA in apoptotic cells. Am. J. Pathol. 152, 897–902.
Didenko, V.V., Boudreaux, D.J., and Baskin, D.S. (1999) Substantial background reduction in ligase-based apoptosis detection using newly designed hairpin oligonucleotide probes. Biotechniques 27, 1130–1132.
Fortuno, M.A., Gonzalez, A., Ravassa, S., Lopez, B., and Diez, J. (2003) Clinical implications of apoptosis in hypertensive heart disease. Am. J. Heart Circ. Physiol. 284, H1495–H1506.
Gonzalez, A., Fortuno, M.A., Querejeta, R., Ravassa, S., Lopez, B., Lopez, N., and Diez, J. (2003) Cardiomyocyte apoptosis in hypertensive cardiomyopathy. Cardiovasc. Res. 59, 549–562.
Kunapuli, S., Rosanio, S., and Schwarz, E.R. (2006) How do cardiomyocytes die? Apoptosis and Autophagic cell death in cardiac myocytes. J. Card. Fail. 12, 381–391.
Koda, M., Takemura, G., Kanoh, M., et al. (2003) Myocytes positive for in situ markers for DNA breaks in human hearts which are hypertrophic, but neither failed nor dilated: a manifestation of cardiac hypertrophy rather than failure. J. Pathol. 199, 229–236.
Hughes, S.E. (2003) Detection of apoptosis using in situ markers for DNA strand breaks in the failing human heart. Fact or epiphenomenon? J. Pathol. 201, 181–186.
Okada, H., Takemura, G., Koda, M., Kanoh, M. Kawase, Y., Minatoguchi, S., and Fujiwara, H. (2005) Myocardial apoptotic index based on in situ DNA nick end-labeling of endomyocardial biopsies does not predict prognosis of dilated cardiomyopathy. Chest 128, 1060–1062.
Jugdutt, B.I., and Idikio, H.A. (2005) Apoptosis and oncosis in acute coronary syndromes: assessment and implications. Mol. Cell. Biochem. 270, 177–200.
Takemura, G., and Fujiwara, H. (2006) Morphological aspects of apoptosis in heart diseases. J. Cell. Mol. Med. 10, 56–75.
Takemura, G., and Fujiwara, H. (2003) Doxorubicin-induced cardiomyopathy from the cardiotoxic mechanisms to management. Prog. Cardiovasc. Dis. 49, 330–352.
Lukes, D.J., Tivesten, A., Wilton, J., Lundgren, A., Rakotonirainy, O., Kjellström, C., Isgaard, J., Karlsson-Parra, A., Soussi, B., and Olausson, M. (2003) Early onset of rejection in concordant hamster xeno hearts display signs of necrosis, but not apoptosis, correlating to the phosphocreatine concentration. Transpl. Immunol. 12, 29–40.
Zhu, C., Qiu, L., Wang, X., Hallin, U., Candé, C., Kroemer, G., Hagberg, H., and Blomgren, K. (2003) Involvement of apoptosis-inducing factor in neuronal death after hypoxia-ischemia in the neonatal rat brain. J. Neurochem. 86, 306–317.
Wang, X., Zhu, C., Qiu, L., Hagberg, H., Sandberg, M., and Blomgren, K. (2003) Activation of ERK1/2 after neonatal rat cerebral hypoxia-ischaemia. J. Neurochem. 86, 351–62.
Plesnila, N., Zhu, C., Culmsee, C., Gröger, M., Moskowitz, M.A., and Blomgren, K. (2004) Nuclear translocation of apoptosis-inducing factor after focal cerebral ischemia. J. Cereb. Blood Flow Metab. 24, 458–466.
Stein, A.B., Bolli, R., Guo, Y., Wang, O.L., Tan, W., Wu, W.J., Zhu, X., Zhu, Y., and Xuan, Y.T. (2007) The late phase of ischemic preconditioning induces a prosurvival genetic program that results in marked attenuation of apoptosis. J. Mol. Cell. Cardiol. 42, 1075–1085.
Lesauskaite, V., Epistolato, M.C., Ivanoviene, L., and Tanganelli, P. (2004) Apoptosis of cardiomyocytes in explanted and transplanted hearts. Comparison of results from in situ TUNEL, ISEL, and ISL reactions. Am. J. Clin. Pathol. 121, 108–116.
Sun, B., Huang, Q., Liu, S., Chen, M., Hawks, C.L., Wang, L., Zhang, C., and Hornsby, P.J. (2004) Progressive loss of malignant behavior in telomerase-negative tumorigenic adrenocortical cells and restoration of tumorigenicity by human telomerase reverse transcriptase. Cancer Res. 64, 6144–6151.
Donath, S., Li, P., Willenbockel, C., Al-Saadi, N., Gross, V., Willnow, T., Bader, M., Martin, U., Bauersachs, J., Wollert, K.C., Dietz, R., and von Harsdorf, R. (2006) Apoptosis repressor with caspase recruitment domain is required for cardioprotection in response to biomechanical and ischemic stress. Circulation. 113, 1203–1212.
Audo, I., Darjatmoko, S.R., Schlamp, C.L., Lokken, J.M., Lindstrom, M.J., Albert, D.M., and Nickells, R.W. (2003) Vitamin D analogues increase p53, p21, and apoptosis in a xenograft model of human retinoblastoma. Invest. Ophthalmol. Vis. Sci. 44, 4192–4199.
Matsuoka, R., Ogawa, K., Yaoita, H., Naganuma, W., Maehara, K., and Maruyama, Y. (2002) Characteristics of death of neonatal rat cardiomyocytes following hypoxia or hypoxia-reoxygenation: the association of apoptosis and cell membrane disintegrity. Heart Vessels 16, 241–248.
Hornsby, P.J., and Didenko, V.V. (2002) In situ DNA ligation as a method for labeling apoptotic cells in tissue sections: an overview, in In Situ Detection of DNA Damage: Methods and Protocols (Didenko, V.V. ed.), Humana, Totowa, NJ, pp.133–141.
Didenko, V.V. (2002) Detection of specific double-strand DNA breaks and apoptosis in situ using T4 DNA ligase, in In Situ Detection of DNA Damage: Methods and Protocols (Didenko, V.V. ed.), Humana, Totowa, NJ, pp.143–151.
Ribeiro, G.F., Côrte-Real, M., and Johansson, B. (2006) Characterization of DNA damage in yeast apoptosis induced by hydrogen peroxide, acetic acid, and hyperosmotic shock. Mol. Biol. Cell. 17, 4584–4591.
Schoppet, M., Al-Fakhri, N., Franke, F.E., Katz, N., Barth, P.J., Maisch, B., Preissner, K.T., and Hofbauer, L.C. (2004) Localization of osteoprotegerin, tumor necrosis factor-related apoptosis-inducing ligand, and receptor activator of nuclear factor-kappaB ligand in Mönckeberg’s sclerosis and atherosclerosis. J. Clin. Endocrinol. Metab. 89, 4104–4112.
Frustaci, A., Chimenti, C., Pieroni, M., Salvatori, L., Morgante, E., Sale, P., Ferretti, E., Petrangeli, E., Gulino, A., and Russo, M.A. (2006) Cell death, proliferation and repair in human myocarditis responding to immunosuppressive therapy. Mod. Pathol. 19, 755–765.
Walker, P.R., Carson, C., Leblanc, J., and Sikorska, M. (2002) Labeling DNA damage with terminal transferase: applicability, specificity and limitations, in In Situ Detection of DNA Damage: Methods and Protocols (Didenko, V.V. ed.), Humana, Totowa, NJ pp.3–19.
Charriaut-Marlangue, C., and Ben-Ari, Y. (1995) A cautionary note on the use of the TUNEL stain to determine apoptosis. Neuroreport 7, 61–64.
Wolvekamp, M.C., Darby, I.A., and Fuller, P.J. (1998) Cautionary note on the use of end-labeling DNA fragments for detection of apoptosis. Pathology 30, 267.
Grasl-Kraupp, B., Ruttkay-Nedecky, B., Koudelka, H., Bukowska, K., Bursch, W., and Schulte-Hermann, R. (1995) In situ detection of fragmented DNA (TUNEL assay) fails to discriminate among apoptosis, necrosis, and autolytic cell death: a cautionary note. Hepatology 21, 1465.
Sloop, G.D., Roa, J.C., Delgado, A.G., Balart, J.T., Hines, M.O., and Hill, J.M. (1999) Histologic sectioning produces TUNEL reactivity. A potential cause of false-positive staining. Arch. Pathol. Lab. Med. 123, 529.
Bassotti, G., Villanacci, V., Fisogni, S., Cadei, M., Galletti, A., Morelli, A., and Salerni, B. (2007) Comparison of three methods to assess enteric neuronal apoptosis in patients with slow transit constipation. Apoptosis 12, 329–332.
Didenko, V.V., Ngo, H., Minchew, C.L., Boudreaux, D.J., Widmayer, M.A, and Baskin, D.S. (2002) Visualization of irreparable ischemic damage in brain by selective labeling of double-strand blunt-ended DNA breaks. Mol. Med. 8, 818–823.
Didenko, V.V., Ngo, H., James, W., and Baskin, D.S. (2003) Early necrotic DNA degradation: presence of blunt ended DNA breaks, 3′ and 5′ overhangs in apoptosis but only 5′ overhangs in necrosis. Am. J. Pathol. 162, 1571–1578.
Sikorska, M., and Walker, P.R. (1998) Endonuclease activities and apoptosis, in When Cells Die (Lockshin, R.A., Zakeri, Z., and Tilly, J.L., eds.), Wiley-Liss, New York, pp.211–242.
Barry, M.A., and Eastman, A. (1993) Identification of deoxyribonuclease II as an endonuclease involved in apoptosis. Arch. Biochem. Biophys. 300, 440–450
Didenko, V.V. (2008) New in apoptosis imaging: dual detection of self-execution and waste-management. Cellutions 1, 13–15.
Grosse, F., and Manns, A. (1993) Terminal deoxyribonucleotidyl transferase (EC 2.7.7.31), in Enzymes of Molecular Biology (Burrell, M.M. ed.), Humana, Totowa, NJ, pp.95–105.
Maunders, M.J. (1993) DNA and RNA ligases (EC 6.5.1.1, EC 6.5.1.2,and EC 6.5.1.3), in Enzymes of Molecular Biology (Burrell, M.M. ed.), Humana, Totowa, NJ, pp.213–230.
Didenko, V.V., Ngo, H., and Baskin, D.S. (2002) In situ detection of double-strand DNA breaks with terminal 5′OH groups, in In Situ Detection of DNA Damage: Methods and Protocols (Didenko, V.V. ed.), Humana, Totowa, NJ, pp.153–159.
Didenko, V.V., Minchew, C.L., Shuman, S., and Baskin, D.S. (2004) Semi-artificial fluorescent molecular machine for DNA damage detection. Nano Lett. 4, 2461–2466.
Didenko, V.V. (2006) Oscillating probe for dual detection of 5′PO4 and 5′OH DNA breaks in tissue sections, in Fluorescent Energy Transfer Nucleic Acid Probes: Methods and Protocols (Didenko, V.V. ed.), Humana, Totowa, NJ, pp.59–69.
Al-Lamki, R.S., Skepper, J.N., Loke, Y.W., King, A., and Burton, G.J. (1998) Apoptosis in the early human placental bed and its discrimination from necrosis using the in-situ DNA ligation technique. Hum. Reprod. 13, 3511–3519.
Donath, S., Li, P., Willenbockel, C., Al-Saadi, N., Gross, V., Willnow, T., Bader, M., Martin, U., Bauersachs, J., Wollert, K.C., Dietz, R., and von Harsdorf, R. (2006) Apoptosis repressor with caspase recruitment domain is required for cardioprotection in response to biomechanical and ischemic stress. Circulation 7, 1203–1212.
Durand, E., Mallat, Z., Addad, F., Vilde, F., Desnos, M., Guérot, C., Tedgui, A., and Lafont, A. (2002) Time courses of apoptosis and cell proliferation and their relationship to arterial remodeling and restenosis after angioplasty in an atherosclerotic rabbit model. J. Am. Coll. Cardiol. 39, 1680–1685.
Kockx, M.M., Muhring, J., Knaapen, M.W.M., and De Meyer, G.R.Y. (1998) RNA synthesis and splicing interferes with DNA in situ end labeling techniques used to detect apoptosis. Am. J. Pathol. 152, 885–888.
Zhu, C., Wang, X., Hagberg, H., and Blomgren, K. (2000) Correlation between caspase-3 activation and three different markers of DNA damage in neonatal cerebral hypoxia-ischemia. J. Neurochem. 75, 819–829.
Watanabe, M., Hitomi, M., van der Wee, K., et al. (2002) The pros and cons of apoptosis assays for use in the study of cells, tissues, and organs. Microsc. Microanal. 8, 375–391.
Murata, I., Takemura, G., Asano, K., Sano, H., Fujisawa, K., Kagawa, T., Baba, K., Maruyama, R., Minatoguchi, S., Fujiwara, T., and Fujiwara, H. (2002) Apoptotic cell loss following cell proliferation in renal glomeruli of Otsuka Long-Evans Tokushima Fatty rats, a model of human type 2 diabetes. Am. J. Nephrol. 22, 587–595.
Zavitsanou, K., Nguyen, V., Greguric, I., Chapman, J., Ballantyne, P., and Katsifis, A. (2007) Detection of apoptotic cell death in the thymus of dexamethasone treated rats using [123I]annexin V and in situ oligonucleotide ligation. J. Mol. Histol. 38, 313–319.
Mahaney, B.L., Meek, K., and Lees-Miller, S.P. (2009) Repair of ionizing radiation-induced DNA double-strand breaks by non-homologous end-joining. Biochem. J. 417, 639–650.
Staley, K., Blaschke, A.J., and Chun, J. (1997) Apoptotic DNA fragmentation is detected by a semi-quantitative ligation-mediated PCR of blunt DNA ends. Cell Death Differ. 4, 66–75.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer Science+Business Media, LLC
About this protocol
Cite this protocol
Hornsby, P.J., Didenko, V.V. (2011). In Situ Ligation: A Decade and a Half of Experience. In: Didenko, V. (eds) DNA Damage Detection In Situ, Ex Vivo, and In Vivo. Methods in Molecular Biology, vol 682. Humana Press, Totowa, NJ. https://doi.org/10.1007/978-1-60327-409-8_5
Download citation
DOI: https://doi.org/10.1007/978-1-60327-409-8_5
Published:
Publisher Name: Humana Press, Totowa, NJ
Print ISBN: 978-1-60327-408-1
Online ISBN: 978-1-60327-409-8
eBook Packages: Springer Protocols