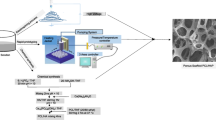
Abstract
Computer-aided tissue engineering approach was used to develop a novel Precision Extrusion Deposition (PED) process to directly fabricate Polycaprolactone (PCL) and composite PCL/Hydroxyapatite (PCL-HA) tissue scaffolds. The process optimization was carried out to fabricate both PCL and PCL-HA (25% concentration by weight of HA) with a controlled pore size and internal pore structure of the 0°/90° pattern. Two groups of scaffolds having 60 and 70% porosity and with pore sizes of 450 and 750 microns, respectively, were evaluated for their morphology and compressive properties using Scanning Electron Microscopy (SEM) and mechanical testing. The surface modification with plasma was conducted on PCL scaffold to increase the cellular attachment and proliferation. Our results suggested that inclusion of HA significantly increased the compressive modulus from 59 to 84 MPa for 60% porous scaffolds and from 30 to 76 MPa for 70% porous scaffolds. In vitro cell–scaffolds interaction study was carried out using primary fetal bovine osteoblasts to assess the feasibility of scaffolds for bone tissue engineering application. In addition, the results in surface hydrophilicity and roughness show that plasma surface modification can increase the hydrophilicity while introducing the nano-scale surface roughness on PCL surface. The cell proliferation and differentiation were calculated by Alamar Blue assay and by determining alkaline phosphatase activity. The osteoblasts were able to migrate and proliferate over the cultured time for both PCL as well as PCL-HA scaffolds. Our study demonstrated the viability of the PED process to the fabricate PCL and PCL-HA composite scaffolds having necessary mechanical property, structural integrity, controlled pore size and pore interconnectivity desired for bone tissue engineering.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Hollister SJ, Maddox RD, Taboas JM (2002) Optimal design and fabrication of scaffolds to mimic tissue properties and satisfy biological constraints. Biomaterials 23:4095–4103
Hutmacher DW (2000) Scaffolds in tissue engineering bone and cartilage. Biomaterials 21:2529–2543
Sun W, Lal P (2002) Recent development on computer aided tissue engineering – a review. Comput Methods Programs Biomed 67:85–103
Sun W, Darling A, Starly B, Nam J (2004) Computer-aided tissue engineering: overview, scope and challenges. Biotechnol Appl Biochem 39:29–47
Sun W, Starly B, Darling A, Gomez C (2004) Computer-aided tissue engineering: application to biomimetic modeling and design of tissue scaffolds. Biotechnol Appl Biochem 39:49–58
Taboas JM, Maddox RD, Krebsbach PH, Hollister SJ (2003) Indirect solid free form fabrication of local and global porous, biomimetic and composite 3D polymer-ceramic scaffolds. Biomaterials 24:181–194
Yang S, Leong K, Du Z, Chua C (2002) The design of scaffolds for use in tissue engineering. Part 2: rapid prototy** techniques. Tissue Eng 8(1):1–11
Simpson RL, Wiria FE, Amis AA, Chua CK, Leong KF, Hansen UN, Chandrasekaran M, Lee MW (2008) Development of a 95/5 poly(l-lactide-co-glycolide)/hydroxylapatite and β-tricalcium phosphate scaffold as bone replacement material via selective laser sintering. J Biomed Mater Res B Appl Biomater 84(1):17–25
Wiria FE, Leong KF, Chua CK, Liu Y (2007) Poly-e-caprolactone/hydroxyapatite for tissue engineering scaffolds fabrication via selective laser sintering. Acta Biomater 3(1):1–12
Tan KH, Chua CK, Leong KF, Cheah CM, Gui WS, Tan WS, Wiria FE (2005) Selective laser sintering of biocompatible polymers for applications in tissue engineering. Biomed Mater Eng 15(1–2):113–124
Chua CK, Leong KF, Tan KH, Wiria FE, Cheah CM (2004) Development of tissue scaffolds using selective laser sintering of polyvinyl alcohol/hydroxyapatite biocomposite for craniofacial and joint defects. J Mater Sci Mater Med 15(10):1113–1121
Venkataraman N (2000) The process-property-performance relationships of feedstock material used for fused deposition of ceramic (FDC). Rutgers University, New Brunswick
McNulty TF, Shanefield DJ, Danforth SC, Safari A (1999) Dispersion of lead zirconate titanate for fused deposition of ceramics. J Am Ceram Soc 8(7):1757–1760
Venkataraman N, Rangarajan S, Matthewson MJ, Harper B, Safari A, Danforth SC et al (2000) Feedstock material property – process relationship in fused deposition of ceramics (FDC). Rapid Prototy** J 5(4):244–252
Darling A, Sun W (2004) 3D microtomographic characterization of precision extruded poly-e-caprolactone tissue scaffolds. J Biomed Mater Res B Appl Biomater 70B(2):311–317
Engelberg I, Kohn J (1991) Physicomechanical properties of degradable polymers used in medical applications: a comparative study. Biomaterials 12(3):292–304
Hutmacher DW, Schantz T, Zein I, Ng KW, Teoh SH, Tan KC (2001) Mechanical properties and cell cultural response of polycaprolactone scaffolds designed and fabricated via fused deposition modeling. J Biomed Mater Res 55:203–216
Wang F, Shor L, Darling A, Khalil S, Sun W, Guceri S et al (2004) Precision extruding deposition and characterization of cellular poly-epsilon-caprolactone tissue scaffolds. Rapid Prototy** J 10(1):42–49
Li WJ, Tuli R, Okafor C, Derfoul A, Danielson KG, Hall DJ et al (2005) A three-dimensional nanofibrous scaffold for cartilage tissue engineering using human mesenchymal stem cells. Biomaterials 26(6):599–609
Karen JLB, Porter S, Kellam JF (2000) Biomaterial developments for bone tissue engineering. Biomaterials 21:2347–2359
Chen G, Zhou P, Mei N, Chen X, Shao Z, Pan L, Wu C (2004) J Materials Sci Mater Med 15:671
Vasilets VN, Kuznetsov AV, Sevast’yanov VI (2006) High energy. Chemistry 40(2):79
Gray H (2000) Anatomy of the human body. In: Lewis WH (ed) New York: BARTLEBY.COM
Bellini A (2002) Fused deposition of ceramics: a comprehensive experimental, analytical and computational study of material behavior, fabrication process and equipment design. Drexel University, Philadelphia
Yildirim ED, Ayan H, Vasilets VN, Fridman A, Guceri S, Sun W (2008) Plasma Process Polym 5:58–66
Owen TA et al (1990) Progressive development of the rat osteoblast phenotype in vitro: reciprocal relationships in expression of genes associated with osteoblast proliferation and differentiation during formation of the bone extracellular matrix. J Cell Physiol 143(3):420–430
Chen G, Zhpu P, Mei N, Chen X, Shao Z (2004) Silk fibroin modified porous poly-epsilon-caprolactone scaffold for human fibroblast culture in vitro. J Mater Sci Mater Med 15:671–677
Oyane A, Uchida M, Yokoyama Y, Choong C, Triffitt J, Ito A (2005) Simple surface modification of poly(epsilon-caprolactone) to induce its apatite-forming ability. J Biomed Mater Res A 75A(1):138–145
Chang G, Absolom D, Strong A, Stubley G, Zingg W (1988) Physical and hydrodynmaic factors affecting erythrocyte adhesion to polymer surfaces. J Biomed Mater Res 22:13–29
Rohl L, Larsen E, Linde F, Odgaard A, Jorgensen J (1991) Tensile and compressive properties of cancellous bone. J Biomech 24(12):1143–1149
Chim H et al (2003) Efficacy of glow discharge gas plasma treatment as a surface modification process for three-dimensional poly (d, l-lactide) scaffolds. J Biomed Mater Res A 65A(3):327–335
Grythe KF, Hansen FK (2006) Langmuir 22:6109
Wu S (1982) Polymer interface and adhesion. Marcell Dekker Inc, New York
Acknowledgment
Support from the National Science Foundation Grant No. 235342 “Computer-Aided Tissue Engineering” to this research is acknowledged.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Springer Science+Business Media, LLC
About this chapter
Cite this chapter
Shor, L., Yildirim, E.D., Güçeri, S., Sun, W. (2010). Precision Extruding Deposition for Freeform Fabrication of PCL and PCL-HA Tissue Scaffolds. In: Narayan, R., Boland, T., Lee, YS. (eds) Printed Biomaterials. Biological and Medical Physics, Biomedical Engineering. Springer, New York, NY. https://doi.org/10.1007/978-1-4419-1395-1_6
Download citation
DOI: https://doi.org/10.1007/978-1-4419-1395-1_6
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4419-1394-4
Online ISBN: 978-1-4419-1395-1
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)