Abstract
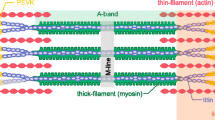
It is now generally accepted that contraction of striated muscle occurs when the thin, actin-containing, and the thick, myosin-containing, filaments slide past each other while the length of both types of filaments remains constant (Huxley and Hanson, 1954; Huxley and Niedergerke, 1954; see Chapter 1). To form a theory of muscle contraction, the mechanism has to be defined which drives this sliding process, leading to muscle shortening at maximum speed when the actin and myosin filaments are allowed to slide freely past each other (unloaded isotonic contraction) or leading to development of maximum force when sliding of filaments is prevented (isometric contraction). Of the many proposed processes that are able to generate force or motion between two interdigitating sets of filaments (for a detailed discussion see Huxley, 1974, 1980), the crossbridge theory of muscle contraction (A. F. Huxley, 1957; H. E. Huxley, 1969) has received general acceptance.
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Adelstein, R. S. and Eisenberg, E. (1980). Regulation and kinetics of the actin-myosin-ATP interaction. Ann. Rev. Biochem., 49, 921–956
Alberty, R. A. (1968). Effect of pH and metal ion concentrations on the equilibrium hydrolysis of adenosine triphosphate to adenosine diphosphate. J. Biol. Chem., 243, 1337–1343
Anderson, M. L. and Schoenberg, M. (1987). Possible cooperativity in crossbridge detachment in muscle fibres having magnesium pyrophosphate at the active site. Biophys. J., 52, 1077–1082
Bagni, M. A., Cecchi, G., Colomo, F. and Tesi, C. (1988). The mechanical characteristics of the contractile machinery at different levels of activation in intact single muscle fibres of the frog. In Sugi, H. and Pollack, G. H. (Eds.), Molecular Mechanisms of Muscle Contraction. Plenum, New York, pp.473–488
Brenner, B. (1983a). Cross-bridge attachment during isotonic shortening in single skinned rabbit psoas fibers. Biophys. J., 41, 33a
Brenner, B. (1983b). Technique for stabilizing the striation pattern in maximally calcium-activated skinned rabbit psoas fibers. Biophys. J., 41, 99–102
Brenner, B. (1984). The rate of force redevelopment in single skinned rabbit psoas fibers. Biophys. J., 45, 155a
Brenner, B. (1985a). Sarcomeric domain organization within single skinned rabbit psoas fibers and its effects on laser light diffraction patterns. Biophys. J., 48, 967–982
Brenner, B. (1985b). Correlation between the cross-bridge cycle in muscle and the actomyosin ATPase cycle in solution, J. Muscle Res. Cell Motil., 6, 659–664
Brenner, B. (1986a). The cross-bridge cycle in muscle. Mechanical, biochemical and structural studies on single skinned rabbit psoas fibers to characterize cross-bridge kinetics in muscle for correlation with the actomyosin ATPase in solution. Basic Res. Cardiol., 81, Suppl. 1, 1–15
Brenner, B. (1986b). Zum molekularen Mechanismus der Muskelkontraktion. Mechanische, biochemische und röntgenstrukturanalytische Untersuchungen am isolierten kontraktilen Apparat von Skelettmuskelfasern. Habilitationsschrift an der Eberhard-Karls-Universität Tübingen
Brenner, B. (1988a). Effect of Ca2+ on cross-bridge turnover kinetics in skinned single rabbit psoas fibres: Implications for regulation of muscle contraction. Proc. Natl Acad. Sci. USA, 85, 3265–3269
Brenner, B. (1988b). An experimental approach to determine cross-bridge turnover kinetics during isometric and isotonic steady state contraction using skinned skeletal muscle fibres of the rabbit. Pflügers Arch., 411, R186
Brenner, B. (1988c). Evidence for rapidly reversible cross-bridge attachment to actin in Ca2+-activated skinned rabbit psoas muscle fibers. Pflügers Arch., 412, R79
Brenner, B., Chalovich, J., Greene, L. E., Eisenberg, E. and Schoenberg, M. (1986a). Stiffness of skinned rabbit psoas fibers in MgATP and MgPPi solution. Biophys. J., 50, 685–691
Brenner, B. and Eisenberg, E. (1986). The rate of force generation in muscle: correlation with actomyosin ATPase in solution. Proc. Natl Acad. Sci. USA, 83, 3542–3546
Brenner, B., Schoenberg, M., Chalovich, J. M., Greene, L. E. and Eisenberg, E. (1982). Evidence for cross-bridge attachment in relaxed muscle at low ionic strength. Proc. Natl Acad. Sci. USA, 79, 7288–7291
Brenner, B. and Squire, J. M. (1987). Rapid stiffness of single relaxed skinned fish muscle fibres: no detectable crossbridge attachment at low ionic strength. J. Muscle Res. Cell Motil., 8, 66–67
Brenner, B. and Yu, L. C. (1985). Equatorial X-ray diffraction from single skinned rabbit psoas fibers at various degrees of activation. Changes in intensities and lattice spacing. Biophys. J., 48, 829–834
Brenner, B., Yu, L. C., Greene, L. E., Eisenberg, E. and Schoenberg, M. (1986b). Ca2+-sensitive cross-bridge dissociation in the presence of MgPPi in skinned rabbit psoas fibers. Biophys. J., 50, 1101–1108
Brenner, B., Yu, L. C. and Podolsky, R. J. (1984). X-ray diffraction evidence for cross-bridge formation in relaxed muscle fibers at various ionic strengths. Biophys. J., 46, 299–306
Bressler, B. H. and Clinch, N. F. (1974). The compliance of contracting skeletal muscle. J. Physiol. (Lond.), 237, 477–493
Cecchi, G., Colomo, F., Lombardi, V. and Piazzesi, G. (1987). Stiffness of frog muscle fibers during rise of tension and relaxation in fixed-end or length-clamped tetani. Pflügers Arch., 409, 39–46
Cecchi, G., Griffiths, P. J. and Taylor, S. (1982). Muscular contraction: kinetics of crossbridge attachment studied by high-frequency stiffness measurements. Science, N.Y., 217, 70–72
Chalovich, J. M., Chock, P. B. and Eisenberg, E. (1981). Mechanism of action of troponin-tropomyosin. J. Biol. Chem., 256, 575–578
Chalovich, J. M. and Eisenberg, E. (1982). Inhibition of actomyosin ATPase activity by troponin-tropomyosin without blocking the binding of myosin to actin. J. Biol. Chem., 257, 2431–2437
Chalovich, J. M., Greene, L. E. and Eisenberg, E. (1989). Crosslinked myosin subfragment 1: A stable analogue of the subfragment ATP complex. Proc. Natl Acad. Sci. USA, 80, 4909–4913
Chalovich, J. M. and Eisenberg, E. (1986). The effect of troponin tropomyosin on the binding of heavy meromyosin to actin in the presence of ATP. J. Biol. Chem., 261, 5088–5093
Cleworth, D. R. and Edman, K. A. P. (1972). Changes in sarcomere length during isometric tension development in frog skeletal muscle. J. Physiol. (Lond.), 227, 1–17
Cooke, R. and Franks, K. (1980). All myosin heads form bonds with actin in rabbit rigor skeletal muscle. Biochemistry, 19, 2265–2269
Cooke, R. and Thomas, D. (1980). Spin label studies of the structure and dynamics of glycerinated muscle fibers: applications. Fed. Proc, 39, 1962
Craig, R., Greene, L. E. and Eisenberg, E. (1985). Structure of the actin-myosin complex in the presence of ATP. Proc. Natl Acad. Sci. USA, 82, 3247–3251
Eastwood, A. B., Wood, D. S., Bock, K. L. and Sorenson, E. E. (1979). Chemically skinned mammalian skeletal muscle I. The structure of skinned rabbit psoas. Tissue Cell, 11, 553–556
Ebashi, S., Endo, M. and Ohtsuki, I. (1969). Control of muscle contraction. Quart. Rev. Biophys., 2, 351–384
Edman, K. A. P. (1979). The velocity of unloaded shortening and its relation to sarcomere length and isometric force in vertebrate muscle fibres. J. Physiol. (Lond.), 291, 143–159
Eisenberg, E. and Greene, L. E. (1980). The relation of muscle biochemistry to muscle physiology. Ann. Rev. Physiol., 42, 293–309
Eisenberg, E. and Hill, T. L. (1978). A cross-bridge model of muscle contraction. Prog. Biophys. Mol. Biol., 33, 55–82
Eisenberg, E. and Hill, T. L. (1985). Muscular contraction and free energy transduction in biological systems. Science, N.Y., 227, 999–1006
Eisenberg, E., Hill, T. L. and Chen, Y. (1980). Cross-bridge model of muscle contraction. Quantitative analysis. Biophys. J., 29, 195–227
Ferenczi, M. A., Homsher, E. and Trentham, D. R. (1984). The kinetics of magnesium adenosine triphosphate cleavage in skinned muscle fibres of the rabbit. J. Physiol. (Lond.), 352, 575–599
Ferenczi, M. A., Simmons, R. M. and Sleep, J. A. (1982). General considerations of cross-bridge models in relation to the dependence on MgATP concentration of mechanical parameters of skinned fibers from frog muscle. In Twarog, B. M., Levine, R. J. C. and Dewey, M. M. (Eds.), Basic Biology of Muscle: A Comparative Approach. Raven Press, New York, p. 91
Ford, L. E., Huxley, A. F. and Simmons, R. M. (1977). Tension responses to sudden length change in stimulated frog muscle fibres near slack length. J. Physiol. (Lond.), 269, 441–515
Ford, L. E., Huxley, A. F. and Simmons, R. M. (1981). The relation between stiffness and filament overlap in stimulated frog muscle fibres. J. Physiol. (Lond.), 311, 219–249
Ford, L. E., Huxley, A. F. and Simmons, R. M. (1986). Tension transients during the rise of tetanic tension in frog muscle fibres. J. Physiol. (Lond.), 372, 595–609
Geeves, M. A., Goody, R. S. and Gutfreund, H. (1984). Kinetics of acto.Sl interaction. J. Muscle Res. Cell Motil., 5, 351–361
Geeves, M. A., Jeffries, T. E. and Millar, N. C. (1986). ATP-induced dissociation of rabbit skeletal actomyosin subfragment 1. Characterization of an isomerization of the ternary acto-S1-ATP complex. Biochemistry, 25, 8454–8458
Goldman, Y. E. (1987). Kinetics of the actomyosin ATPase in muscle fibers. Ann. Rev. Physiol., 49, 637–654
Goldman, Y. E. and Hibberd, M. G. (1983). Ca2+-dependence of tension transients initiated by photolysis of caged adenosine triphosphate in rabbit skinned muscle fibres. J. Physiol. (Lond.), 341, 38P
Goldman, Y. E., Hibberd, M. G. and Trentham, D. R. (1984a). Relaxation of rabbit psoas muscle fibres from rigor by photochemical generation of adenosine-5-triphosphate. J. Physiol. (Lond.), 354, 577–604
Goldman, Y. E., Hibberd, M. G. and Trentham, D. R. (1984b). Initiation of active contraction by photogeneration of adenosine-5′-triphosphate in rabbit psoas muscle fibres. J. Physiol. (Lond.), 354, 605–624
Goldman, Y. E., McCray, J. A. and Ranatunga, K. W. (1987). Transient tension changes initiated by laser temperature jumps in rabbit psoas muscle fibres. J. Physiol. (Lond.), 392, 71–95
Goldman, Y. E. and Simmons, R. M. (1977). Active and rigor muscle stiffness. J. Physiol. (Lond.), 269, 55P–57P
Goldman, Y. E. and Simmons, R. M. (1984). Control of sarcomere length in skinned muscle fibres of Rana temporaria during mechanical transients. J. Physiol. (Lond.), 350, 497–518
Goody, R. S., Hofmann, W. and Mannherz, H. G. (1977). The binding constant of ATP to myosin Sl fragment. Eur. J. Biochem., 78, 317–324
Gordon, A. M., Huxley, A. F. and Julian, F. J. (1966). The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J. Physiol. (Lond.), 184, 170–192; see also Figure 1.14, p. 22
Green, L. E. and Eisenberg, E. (1980a). Dissociation of the actin subfragment-one complex by adenyl-5′-yl imidodiphosphate, ADP, and PPi. J. Biol. Chem., 255, 543–548
Greene, L. E. and Eisenberg, E. (1980b). Cooperative binding of myosin subfragment-1 to the actin-troponin-tropomyosin complex. Proc. Natl Acad. Sci. USA, 77, 2616–2620
Greene, L. E. and Eisenberg, E. (1982). Interaction of actin and myosin in the presence and absence of ATP. Methods of Enzymology, 85, pp. 709–724
Greene, L. E., Sellers, J. R., Eisenberg, E. and Adelstein, R. S. (1983). Binding of gizzard smooth muscle myosin subfragment-one to actin in the presence and absence of ATP. Biochemistry, 22, 530–535
Gutfreund, H. (1972). Enzymes: Physical Principles. Wiley Interscience, London
Haselgrove, J. C. (1973). X-ray evidence for the conformational change in the actin-containing filaments of vertebrate striated muscle. Cold Spring Harbor Symp. Quant. Biol., 37, 341–352
Haselgrove, J. C. (1975). X-ray evidence for conformational changes in the myosin filaments of vertebrate striated muscle. J. Mol. Biol., 92, 113–143
Haselgrove, J. C. and Huxley, H. E. (1973). X-ray evidence for radial cross-bridge movement and for the sliding filament model in actively contracting skeletal muscle. J. Mol. Biol., 77, 549–568
Hibberd, M. G., Dantzig, J. A., Trentham, D. R. and Goldman, Y. E. (1985). Phosphate release and force generation in skeletal muscle fibers. Science, N. Y., 228, 1317–1319
Hibberd, M. G. and Trentham, D. R. (1986). Relationships between chemical and mechanical events during muscular contraction. Ann. Rev. Biophys. Biophys. Chem., 15, 119–161
Highsmith, S. (1977). The effects of temperature and salts on myosin subfragment-1 and F-actin association. Arch. Biochem. Biophys., 180, 404–408
Hill, A. V. (1964). The effect of load on the heat of shortening of muscle. Proc. Roy. Soc. Lond., Ser. B, 159, 297–318
Hill, T. L. (1968). On the sliding filament model of muscular contraction, II. Proc. Natl Acad. Sci. USA, 61, 98–105
Hill, T. L. (1974). Theoretical formalism for the sliding filament model of contraction of striated muscle. Part I. Prog. Biophys. Mol. Biol., 28, 267–340
Hill, T. L. (1975). Theoretical formalism for the sliding filament model of contraction of striated muscle. Part II. Prog. Biophys. Mol. Biol., 29, 105–159
Hill, T. L., Eisenberg, E. and Chalovich, J. M. (1981). Theoretical models for cooperative steady-state ATPase activity of myosin subfragment-1 on regulated actin. Biophys. J., 35, 99–112
Huxley, A. F. (1957). Muscle structure and theories of contraction. Prog. Biophys. Biophys. Chem., 7, 255–318
Huxley, A. F. (1973). A note suggesting that the cross-bridge attachment during muscle contraction may take place in two stages. Proc. Roy. Soc. Lond., Ser. B, 183, 83–86
Huxley, A. F. (1974). Muscular contraction. J. Physiol. (Lond.), 243, 1–43
Huxley, A. F. (1980). Reflections on Muscle. Princeton University Press
Huxley, A. F. and Julian, F. J. (1964). Speed of unloaded shortening in frog striated muscle fibres. J. Physiol. (Lond.), 177, 60P–61P
Huxley, A. F., Lombardi, V. and Peachey, L. D. (1981). A system for fast recording of longitudinal displacement of a striated muscle fibre. J. Physiol. (Lond.), 317, 12P–13P
Huxley, A. F. and Niedergerke, R. (1954). Interference microscopy of living muscle fibres. Nature, 173, 971–974
Huxley, A. F. and Simmons, R. M. (1971a). Mechanical properties of cross-bridges of frog striated muscle. J. Physiol. (Lond.), 218, 59P
Huxley, A. F. and Simmons R. M. (1971b). Proposed mechanism of force generation in striated muscle. Nature, 233, 533–538
Huxley, A. F. and Simmons R. M. (1973). Mechanical transients and the origin of muscular force. Cold Spring Harbor Symp. Quant. Biol., 37, 669–680
Huxley, H. E. (1953). Electron microscope studies of the organization of the filaments in striated muscle. Biochim. Biophys. Acta, 12, 387–394
Huxley, H. E. (1957). The double array of filaments in cross-striated muscle. J. Biophys. Biochem. Cytol., 3, 631–647
Huxley, H. E. (1968). Structural differences between resting and rigor muscle. Evidence from intensity changes in the low-angle equatorial x-ray diagram. J. Mol. Biol., 37, 507–520
Huxley, H. E. (1969). The mechanism of muscular contraction. Science, N. Y., 164, 1356–1366
Huxley, H. E. (1973). Structural changes in the actin- and myosin-containing filaments during contraction. Cold Spring Harbor Symp. Quant. Biol., 37, 361–376
Huxley, H. E. (1979). Time resolved X-ray diffraction studies on muscle. In Sugi, H. and Pollack, G. H. (Eds.), Cross-bridge Mechanism in Muscle Contraction. University Park Press, Baltimore, p. 391
Huxley, H. E. and Brown, W. (1967). The low-angle X-ray diagram of vertebrate striated muscle and its behaviour during contraction and rigor. J. Mol. Biol., 30, 383–434
Huxley, H. E. and Hanson, J. (1954). Changes in the cross-striations of muscle during contraction and stretch and their interpretation. Nature, 173, 973–976
Huxley, H. E. and Kress, M. (1985). Crossbridge behaviour during muscle contraction. J. Muscle Res. Cell Motil., 6, 153–161
Julian, F. J. (1969). Activation in a skeletal muscle model with a modification for insect fibrillar muscle. Biophys. J., 9, 547–570
Kimura, M. and Tawada, K. (1984). Is the SII portion of the cross-bridge in glycerinated rabbit psoas fibers compliant in the rigor state? Biophys. J., 45, 603–610
Kress, M., Huxley, H. E., Faruqi, A. R. and Hendrix, J. (1986). Structural changes during activation of frog muscle studied by time-resolved X-ray diffraction. J. Mol. Biol., 188, 325–342
Kuhn, H. J. (1978). Cross-bridge slippage induced by the ATP analogue AMP-PNP and stretch in glycerol-extracted fibrillar muscle fibres. Biophys. Struct. Mech., 4, 159–168
Kuhn, H. J., Güth, K., Drexler, B., Berberich, W. and Rüegg, J. C. (1979). Investigation of the temperature dependence of the cross bridge parameters for attachment, force generation and detachment deduced from mechano-chemical studies in glycerinated single fibres from the dorsal longitudinal muscle of Lethocerus maximus. Biophys. Struct. Mech., 6, 1–29
Kushmerick, M. J. and Davies, R. E. (1969). The chemical energetics of muscle contraction. II. The chemistry, efficiency and power of maximally working sartorius muscles. Proc. Roy. Soc. Lond., Ser. B, 174, 315–353
Kushmerick, M. J. and Krasner, B. (1982). Force and ATPase rate in skinned skeletal muscle fibers. Fed. Proc., 41, 2232–2237
Lovell, S. J. and Harrington, W. F. (1981). Measurement of the fraction of myosin heads bound to actin in rabbit skeletal myofibrils in rigor. J. Mol. Biol., 149, 659–674
Lowey, S. and Luck, S. M. (1969). Equilibrium binding of ADP to myosin. Biochemistry, 8, 3195–3199
Lymn, R. W. and Taylor, E. W. (1971). Mechanism of adenosine triphosphate hydrolysis by actomyosin. Biochemistry, 10, 4617–4624
Magid, A. and Reedy, M. (1980). X-ray diffraction observations of chemically skinned frog skeletal muscle processed by an improved method. Biophys. J., 30, 27–40
Margossian, S. S. and Lowey, S. (1978). Interaction of myosin subfragments with F-actin. Biochemistry, 17, 5431–5439
Marston, S. B. (1973). The nucleotide complexes of myosin in glycerol-extracted muscle fibers. Biochim. Biophys. Acta, 305, 397–412
Marston, S. B. (1982). The rates of formation and dissociation of actin-myosin complexes. Biochem. J., 230, 453–460
Marston, S. B. and Tregear, R. T. (1972). Evidence for a complex between myosin and ADP in relaxed muscle fibers. Nature New Biol., 235, 23–24
Marston, S. B. and Weber, A. (1975). The dissociation constant of the actin-heavy meromyosin subfragment-1 complex. Biochemistry, 14, 3868–3873
Matsubara, I., Goldman, Y. E. and Simmons, R. M. (1984). Changes in the lateral filament spacing of skinned muscle fibres when cross-bridges attach. J. Mol. Biol., 173, 13–33
Matsuda, T. and Podolsky, R. J. (1984). X-ray evidence for two structural states of the actomyosin cross-bridge in muscle fibers. Proc. Natl Acad. Sci. USA, 81, 2364–2368
Natori, R. (1954). The property and contraction process of isolated myofibrils. Jikeikai Med. J., 1, 119–126
Offer, G. and Elliott, A. (1978). Can a myosin molecule bind to two actin filaments? Nature, 271, 325–329
Parry, D. A. D. and Squire, J. M. (1973). Structural role of tropomyosin in muscle regulation: analysis of the x-ray diffraction patterns from relaxed and contracting muscles. J. Mol. Biol., 75, 33–55
Podolsky, R. J. (1960). Kinetics of muscular contraction: the approach to the steady state. Nature, 188, 666–668
Podolsky, R. J. and Nolan, A. C. (1973). Muscle contraction transients, cross-bridge kinetics, and the Fenn effect. Cold Spring Harbor Symp. Quant. Biol., 37, 661–668
Podolsky, R. J. and Teichholz, L. E. (1970). The relation between calcium and contraction kinetics in skinned muscle fibres. J. Physiol. (Lond.), 211, 19–35
Pringle, J. W. S. (1967). The contractile mechanism of insect fibrillar muscle. Prog. Biophys., 17, 1–60
Ramsey, R. W. and Street, S. F. (1940). The isometric length-tension diagram of isolated skeletal muscle fibers of the frog. J. Cell Comp. Physiol., 15, 11–34
Reedy, M. K., Holmes, K. C. and Tregear, R. T. (1965). Induced changes in orientation of the cross-bridges of glycerinated insect flight muscle. Nature (Lond.), 207, 1276–1280
Rosenfeld, S. S. and Taylor, E. W. (1984). The ATPase mechanism of skeletal and smooth muscle acto-subfragment 1. J. Biol Chem., 259, 11908–11919
Rosenfeld, S. S. and Taylor, E. W. (1987a). The mechanism of regulation of actomyosin subfragment 1 ATPase. J. Biol Chem., 262, 9984–9993
Rosenfeld, S. S. and Taylor, E. W. (1987b). The dissociation of 1-N-6-ethenoadenosine diphosphate from regulated actomyosin subfragment 1. J. Biol Chem., 262, 9994–9999
Rudel, R. and Zite-Ferenczy, F. (1979). Interpretation of light diffraction of cross-striated muscle as Bragg reflexion of light by the lattice of contractile proteins. J. Physiol (Lond.), 290, 317–330
Schoenberg, M. (1985). Equilibrium muscle cross-bridge behaviour: theoretical considerations. Biophys. J., 48, 467–475
Schoenberg, M. (1988). Characterization of the myosin adenosine triphosphate (M:ATP) crossbridge in rabbit and frog skeletal muscle fibers. Biophy. J., 54, 135–148
Schoenberg, M., Brenner, B., Chalovich, J. M., Greene, L. E. and Eisenberg, E. (1984). Cross-bridge attachment in relaxed muscle. In Pollack, G. H. and Sugi, H. (Eds.), Contractile Mechanisms in Muscle. Plenum Press, New York, pp. 269–284
Schoenberg, M. and Eisenberg, E. (1985). Muscle cross-bridge kinetics in rigor and in the presence of ATP analogues. Biophys. J., 48, 863–871
Schoenberg, M. and Wells, J. B. (1984). Stiffness, force and sarcomere shortening during a twitch in frog semitendinosus muscle bundles. Biophys. J., 45, 389–397
Simmons, R. M. and Jewell, B. R. (1974). Mechanics and models of muscular contraction. Recent Adv. Physiol, 31, 87–147
Sleep, J. A. and Hutton, R. L. (1978). Actin mediated release of ATP from a myosin-ATP complex. Biochemistry, 17, 5423–5430
Sleep, J. A. and Hutton, R. L. (1980). Exchange between inorganic phosphate and adenosine 5′-triphosphate in the medium by actomyosin subfragment 1. Biochemistry, 19, 1276–1283
Sleep, J. A. and Smith, S. J. (1981). Actomyosin ATPase and muscle contraction. Curr. Top. Bioenerg., 11, 239–286
Squire, J. M. (1972). General model of myosin filament structure. II. Myosin filaments and cross-bridge interactions in vertebrate striated and insect flight muscle. J. Mol. Biol 72, 125–138
Squire, J. M. (1981). The Structural Basis of Muscular Contraction. Plenum Press, New York
Squire, J. M. and Harford, J. J. (1988). Actin filament organization and myosin head labelling patterns in vertebrate skeletal muscles in the rigor and weak binding states. J. Muscle Res. Cell Motil., 9, 344–358
Squire, J. M., Podolsky, R. J., Yu, L. C. and Brenner, B. (1987). Equatorial X-ray diffraction from resting skinned single fibres of fish muscle: little evidence for crossbridge attachment at low ionic strength. J. Muscle Res. Cell Motil., 8, 66
Stein, L. A., Chock, P. B. and Eisenberg, E. (1984). The rate-limiting step in the actomyosin adenosinetriphosphatase cycle. Biochemistry, 23, 1555–1563
Stein, L. A., Schwarz, R. P., Chock, P. B. and Eisenberg, E. (1979). Mechanism of actomyosin adenosine triphosphatase. Evidence that adenosine 5′-triphosphate hydrolysis can occur without dissociation of the actomyosin complex. Biochemistry, 18, 3895–3909
Sundell, C. L., Goldman, Y. E. and Peachey, L. D. (1986). Fine structure in near-field and far-field laser diffraction patterns from skeletal muscle fibers. Biophys J., 49, 521–530
Szent-Györgyi, A. (1949). Free-energy relations and contractions of actomyosin. Biol. Bull., 96, 140–161
Szent-Györgyi, A. G. (1953). Meromyosins, the subunits of myosin. Arch. Biochem. Biophys., 42, 305–320
Tamura, Y., Hatta, I., Matsuda, T., Sugi, H. and Tsuchiya, T. (1982). Changes in muscle stiffness during contraction recorded using ultrasonic waves. Nature, 299, 631–633
Tawada, K. and Kimura, M. (1986). Stiffness of carbodiimide-crosslinked glycerinated muscle fibres in rigor and relaxing solutions at high salt concentrations. J. Muscle Res. Cell Motil., 7, 339–350
Taylor, E. W. (1979). Mechanism of actomyosin ATPase and the problem of muscle contraction. CRC Crit. Rev. Biochem., 6, 103–164
Thomas, D. D. (1987). Spectroscopic probes of muscle cross-bridge action. Ann. Rev. Physiol., 49, 691–709
Tözeren, A. (1988). The influence of doubly-attached cross-bridges on mechanical behaviour of skeletal muscle fibers under equilibrium conditions. Biophys. J., 53, 60a
Tözeren, A. and Schoenberg, M. (1986). The effect of cross-bridge clustering and head-head competition on the mechanical response of skeletal muscle under equilibrium conditions. Biophys. J., 50, 875–884
Trentham, D. R., Eccleston, J. F. and Bagshaw, C. R. (1976). Kinetic analysis of ATPase mechanisms. Quart. Rev. Biophys., 9, 217–281
Trybus, K. M. and Taylor, E. W. (1980). Kinetic studies of the cooperative binding subfragment 1 to regulated actin. Proc. Natl Acad. Sci. USA, 77, 7209–7213
Wagner, P. D. (1984). Effect of skeletal muscle myosin light chain 2 on the Ca2+-sensitive interaction of myosin and heavy meromyosin with regulated actin. Biochemistry, 23, 5950–5956
Wagner, P. D. and Giniger, E. (1981). Calcium-sensitive binding of heavy meromyosin to regulated actin in the presence of ATP. J. Biol. Chem., 256, 12647–12650
Webb, M. R., Hibberd, M. G., Goldman, Y. E. and Trentham, D. R. (1986). Oxygen exchange between Pi in the medium and water during ATP hydrolysis mediated by skinned fibres from rabbit skeletal muscle: Evidence for Pi binding to a force generating state. J. Biol. Chem., 261, 15557–15564
Webb, M. R. and Trentham, D. R. (1981). The mechanism of ATP hydrolysis catalyzed by myosin and actomyosin using rapid reaction techniques to study oxygen exchange. J. Biol. Chem., 256, 10910–10916
White, H. D. (1982). Special instrumentation and techniques for kinetic studies of contractile systems. Methods of Enzymology, 85, pp. 698–708
White, H. D. and Taylor, E. W. (1976). Energetics and mechanism of actomyosin adenosine triphosphatase. Biochemistry, 15, 5818–5826
Williams, D. L., Greene, L. E. and Eisenberg, E. (1988). Cooperative turning on of myosin subfragment 1 adenosinetriphosphate activity by the troponin-tropomyosin-actin complex. Biochem., 27, 6987–6993
Wolcott, R. G. and Boyer, P. D. (1974). The reversal of the myosin and actomyosin ATPase reactions and the free energy of ATP binding to myosin. Biochem. Biophys. Res. Commun., 57, 709–716
Wolcott, R. G. and Boyer, P. D. (1975). Isotopic probes of catalytic steps of myosin adenosine triphosphatase. J. Supramol. Struct., 3, 154–161
Xu, S. G., Kress, M. and Huxley, H. E. (1987). X-ray diffraction studies of the structural state of cross-bridges in skinned frog sartorius muscle at low ionic strength. J. Muscle Res. Cell Motil., 8, 39–54
Yanagida, T., Arata, T. and Oosawa, F. (1985). Sliding distance of actin filament induced by a myosin crossbridge during one ATP hydrolysis cycle. Nature, 316, 366–369
Yanagida, T., Kuranaga, I. and Inoue, A. (1982). Interaction of myosin with thin filaments during contraction and relaxation: Effect of ionic strength. J. Biochem., 92, 407–412
Yu, L. C. and Brenner, B. (1986). High resolution equatorial x-ray diffraction from single skinned rabbit psoas fibers. Biophys. J., 49, 133–135
Yu, L. C. and Brenner, B. (1987). Equatorial X-ray diffraction from fully Ca2+ activated single muscle fibers at low ionic strengths. Biophys. J., 51, 473a
Yu, L. C. and Brenner, B. (1989). Structures of actomyosin crossbridges in relaxed and rigor muscle fibers. Biophys. J., 55, 441–453
Yu, L. C., Hartt, J. E. and Podolsky, R. J. (1979). Equatorial x-ray intensities and isometric force levels in frog sartorius muscle. J. Mol. Biol., 132, 53–67
Editor information
Editors and Affiliations
Copyright information
© 1990 The editor and contributors
About this chapter
Cite this chapter
Brenner, B. (1990). Muscle Mechanics and Biochemical Kinetics. In: Squire, J.M. (eds) Molecular Mechanisms in Muscular Contraction. Topics in Molecular and Structural Biology. Palgrave, London. https://doi.org/10.1007/978-1-349-09814-9_4
Download citation
DOI: https://doi.org/10.1007/978-1-349-09814-9_4
Publisher Name: Palgrave, London
Print ISBN: 978-1-349-09816-3
Online ISBN: 978-1-349-09814-9
eBook Packages: EngineeringEngineering (R0)