Abstract
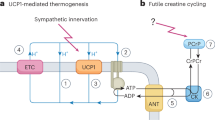
Thermogenic adipose tissue plays a vital function in regulating whole-body energy expenditure and nutrient homeostasis due to its capacity to dissipate chemical energy as heat, in a process called non-shivering thermogenesis. A reduction of creatine levels in adipocytes impairs thermogenic capacity and promotes diet-induced obesityKazak et al, Cell 163, 643–55, 2015; Kazak et al, Cell Metab 26, 660–671.e3, 2017; Kazak et al, Nat Metab 1, 360–370, 2019). Mechanistically, thermogenic respiration can be promoted by the liberation of an excess quantity of ADP that is dependent on addition of creatine. A model of a two-enzyme system, which we term the Futile Creatine Cycle, has been posited to support this thermogenic action of creatine. Futile creatine cycling can be monitored in purified mitochondrial preparations wherein creatine-dependent liberation of ADP is monitored through the measurement of oxygen consumption under ADP-limiting conditions. The current model proposes that, in thermogenic fat cells, mitochondria-targeted creatine kinase B (CKB) uses mitochondrial-derived ATP to phosphorylate creatine (Rahbani JF, Nature 590, 480–485, 2021). The creatine kinase reaction generates phosphocreatine and ADP, and ADP stimulates respiration. Next, a pool of mitochondrial phosphocreatine is directly hydrolyzed by a phosphatase, to regenerate creatine. The liberated creatine can then engage mitochondrial CKB to trigger another round of this cycle to support ADP-dependent respiration. In this model, the coordinated action of creatine phosphorylation and phosphocreatine hydrolysis triggers a futile cycle that produces a molar excess of mitochondrial ADP to promote thermogenic respiration (Rahbani JF, Nature 590, 480–485, 2021; Kazak and Cohen, Nat Rev Endocrinol 16, 421–436, 2020). Here, we provide a detailed method to perform respiratory measurements on isolated mitochondria and calculate the stoichiometry of creatine-dependent ADP liberation. This method provides a direct measure of the futile creatine cycle.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Ward ZJ et al (2019) Projected U.S. state-level prevalence of adult obesity and severe obesity. N Engl J Med 381(25):2440–2450
Ligibel JA et al (2014) American Society of Clinical Oncology position statement on obesity and cancer. J Clin Oncol 32(31):3568–3574
Global BMIMC et al (2016) Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet 388(10046):776–786
Speakman JR (2007) A nonadaptive scenario explaining the genetic predisposition to obesity: the “predation release” hypothesis. Cell Metab 6(1):5–12
Foster DO, Frydman ML (1978) Brown adipose tissue: the dominant site of nonshivering thermogenesis in the rat. Exp Suppl 32:147–151
Cannon B, Nedergaard J (2004) Brown adipose tissue: function and physiological significance. Physiol Rev 84(1):277–359
Chouchani ET, Kazak L, Spiegelman BM (2019) New advances in adaptive thermogenesis: UCP1 and beyond. Cell Metab 29(1):27–37
Roesler A, Kazak L (2020) UCP1-independent thermogenesis. Biochem J 477(3):709–725
Din MU et al (2018) Postprandial oxidative metabolism of human Brown fat indicates thermogenesis. Cell Metab 28(2):207–216.e3
Carpentier AC et al (2018) Brown adipose tissue energy metabolism in humans. Front Endocrinol (Lausanne) 9:447
Cypess AM et al (2009) Identification and importance of brown adipose tissue in adult humans. N Engl J Med 360(15):1509–1517
Cypess AM et al (2015) Activation of human brown adipose tissue by a beta3-adrenergic receptor agonist. Cell Metab 21(1):33–38
Schrauwen P, van Marken Lichtenbelt WD (2016) Combatting type 2 diabetes by turning up the heat. Diabetologia 59(11):2269–2279
van Marken Lichtenbelt WD, Schrauwen P (2011) Implications of nonshivering thermogenesis for energy balance regulation in humans. Am J Physiol Regul Integr Comp Physiol 301(2):R285–R296
van Marken Lichtenbelt WD et al (2009) Cold-activated brown adipose tissue in healthy men. N Engl J Med 360(15):1500–1508
Virtanen KA et al (2009) Functional brown adipose tissue in healthy adults. N Engl J Med 360(15):1518–1525
Betz MJ, Enerback S (2018) Targeting thermogenesis in brown fat and muscle to treat obesity and metabolic disease. Nat Rev Endocrinol 14(2):77–87
Saito M et al (2009) High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 58(7):1526–1531
Bartelt A et al (2011) Brown adipose tissue activity controls triglyceride clearance. Nat Med 17(2):200–205
Berbee JF et al (2015) Brown fat activation reduces hypercholesterolaemia and protects from atherosclerosis development. Nat Commun 6:6356
Fischer AW et al (2020) Brown adipose tissue lipoprotein and glucose disposal is not determined by thermogenesis in uncoupling protein 1-deficient mice. J Lipid Res 61(11):1377–1389
Becher T et al (2020) Brown adipose tissue is associated with improved Cardiometabolic health and regulates blood pressure. bioRxiv, 2020.02.08.933754
Blondin DP et al (2014) Increased brown adipose tissue oxidative capacity in cold-acclimated humans. J Clin Endocrinol Metab 99(3):E438–E446
Lee P et al (2014) Temperature-acclimated brown adipose tissue modulates insulin sensitivity in humans. Diabetes 63(11):3686–3698
Matsushita M et al (2014) Impact of brown adipose tissue on body fatness and glucose metabolism in healthy humans. Int J Obes 38(6):812–817
Mitchell P (1961) Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature 191:144–148
Mills EL et al (2018) Accumulation of succinate controls activation of adipose tissue thermogenesis. Nature 560(7716):102–106
Yoneshiro T et al (2019) BCAA catabolism in brown fat controls energy homeostasis through SLC25A44. Nature 572(7771):614–619
Rahbani JF et al (2021) Creatine kinase B controls futile creatine cycling in thermogenic fat. Nature 590(7846):480–485
Sun Y et al (2021) Mitochondrial TNAP controls thermogenesis by hydrolysis of phosphocreatine. Nature 593(7860):580–585
Watt IN et al (2010) Bioenergetic cost of making an adenosine triphosphate molecule in animal mitochondria. Proc Natl Acad Sci U S A 107(39):16823–16827
Kazak L, Cohen P (2020) Creatine metabolism: energy homeostasis, immunity and cancer biology. Nat Rev Endocrinol 16(8):421–436
Kazak L et al (2015) A creatine-driven substrate cycle enhances energy expenditure and thermogenesis in beige fat. Cell 163(3):643–655
Bertholet AM et al (2017) Mitochondrial patch clamp of beige adipocytes reveals UCP1-positive and UCP1-negative cells both exhibiting futile Creatine cycling. Cell Metab 25(4):811–822.e4
Acknowledgments
We thank Peter Rank (Rank Brothers Limited) for granting permission to use Fig. 1. Funding: Supported by Canadian Institutes of Health Research grant (PJT-159529) and Canadian Foundation for Innovation John R. Evans Leaders Fund (37919) (to L.K.); Canderel and Charlotte and Leo Karassik fellowships (to J.F.R.).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Rahbani, J.F., Chouchani, E.T., Spiegelman, B.M., Kazak, L. (2022). Measurement of Futile Creatine Cycling Using Respirometry. In: Guertin, D.A., Wolfrum, C. (eds) Brown Adipose Tissue. Methods in Molecular Biology, vol 2448. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-2087-8_10
Download citation
DOI: https://doi.org/10.1007/978-1-0716-2087-8_10
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-2086-1
Online ISBN: 978-1-0716-2087-8
eBook Packages: Springer Protocols