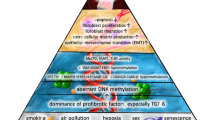
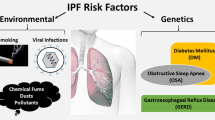
Abstract
Idiopathic pulmonary fibrosis (IPF) is a fatal variation of the interstitial pulmonary illness characterised by extracellular matrix deposition that leads to secretion of inflammatory cytokines and causes fibrosis in the lungs. Further progression of fibrosis leads to cancerous stage of lungs and death. IPF is the worst pathological condition to be focused on and explored because of its rising prevalence, poor prognosis, and inadequate treatment. Even though the disease’s origin is still unknown, several genetic, environmental, and underlying pulmonary problems could set off a number of molecular pathways which is involved in the development of IPF. However, several genetic loci and genetic polymorphisms linked to IPF have been examined by genome-wide association studies and whole-genome sequencing. The newly found gene may clarify key elements in the identification, prognosis, and treatments of IPF. Additionally IPF can result from a variety of epigenetic alternations, including modification of histone, methylation of DNA, and non-coding RNA. This book chapter summarises the pathogenesis of pulmonary fibrosis, available treatment and the pathways that involved in IPF progression and may develop into lung cancer. Furthermore, this highlights epigenetic and molecular mechanism of IPF progression.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Martinez FJ, Collard HR, Pardo A, Raghu G, Richeldi L, Selman M, et al. Idiopathic pulmonary fibrosis. Nat Rev Dis Primers. 2017;3:17074. https://doi.org/10.1038/nrdp.2017.74.
Richeldi L, Collard HR, Jones MG. Idiopathic pulmonary fibrosis. Lancet. 2017;389(10082):1941–52. https://doi.org/10.1016/s0140-6736(17)30866-8.
Lederer DJ, Martinez FJ. Idiopathic pulmonary fibrosis. N Engl J Med. 2018;378(19):1811–23. https://doi.org/10.1056/NEJMra1705751.
Spagnolo P, Kropski JA, Jones MG, Lee JS, Rossi G, Karampitsakos T, et al. Idiopathic pulmonary fibrosis: disease mechanisms and drug development. Pharmacol Ther. 2021;222:107798. https://doi.org/10.1016/j.pharmthera.2020.107798.
Sgalla G, Iovene B, Calvello M, Ori M, Varone F, Richeldi L. Idiopathic pulmonary fibrosis: pathogenesis and management. Respir Res. 2018;19(1):32. https://doi.org/10.1186/s12931-018-0730-2.
Wolters PJ, Collard HR, Jones KD. Pathogenesis of idiopathic pulmonary fibrosis. Annu Rev Pathol. 2014;9:157–79. https://doi.org/10.1146/annurev-pathol-012513-104706.
Maher TM, Bendstrup E, Dron L, Langley J, Smith G, Khalid JM, et al. Global incidence and prevalence of idiopathic pulmonary fibrosis. Respir Res. 2021;22(1):197. https://doi.org/10.1186/s12931-021-01791-z.
Woodcock HV, Maher TM. The treatment of idiopathic pulmonary fibrosis. F1000Prime Rep. 2014;6:16. https://doi.org/10.12703/P6-16.
Mustafin RN. Molecular genetics of idiopathic pulmonary fibrosis. Vavilovskii Zhurnal Genet Selektsii. 2022;26(3):308–18. https://doi.org/10.18699/VJGB-22-37.
Leung J, Cho Y, Lockey RF, Kolliputi N. The role of aging in idiopathic pulmonary fibrosis. Lung. 2015;193(4):605–10. https://doi.org/10.1007/s00408-015-9729-3.
Samarelli AV, Masciale V, Aramini B, Coló GP, Tonelli R, Marchioni A, et al. Molecular mechanisms and cellular contribution from lung fibrosis to lung cancer development. Int J Mol Sci. 2021;22(22) https://doi.org/10.3390/ijms222212179.
Kinoshita T, Goto T. Molecular mechanisms of pulmonary fibrogenesis and its progression to lung cancer: a review. Int J Mol Sci. 2019;20(6) https://doi.org/10.3390/ijms20061461.
Karampitsakos T, Tzilas V, Tringidou R, Steiropoulos P, Aidinis V, Papiris SA, et al. Lung cancer in patients with idiopathic pulmonary fibrosis. Pulm Pharmacol Ther. 2017;45:1–10. https://doi.org/10.1016/j.pupt.2017.03.016.
Ozawa Y, Suda T, Naito T, Enomoto N, Hashimoto D, Fujisawa T, et al. Cumulative incidence of and predictive factors for lung cancer in IPF. Respirology. 2009;14(5):723–8. https://doi.org/10.1111/j.1440-1843.2009.01547.x.
Goto T, Maeshima A, Akanabe K, Oyamada Y, Kato R. Acute exacerbation of idiopathic pulmonary fibrosis of microscopic usual interstitial pneumonia pattern after lung cancer surgery<en-aut-mei> </en-aut-mei>. Ann Thorac Cardiovasc Surg. 2011;17(6):573–6. https://doi.org/10.5761/atcs.cr.10.01619.
Goto T, Maeshima A, Oyamada Y, Kato R. Idiopathic pulmonary fibrosis as a prognostic factor in non-small cell lung cancer. Int J Clin Oncol. 2014;19(2):266–73. https://doi.org/10.1007/s10147-013-0566-1.
Goto T. Measuring surgery outcomes of lung cancer patients with concomitant pulmonary fibrosis: a review of the literature. Cancers. 2018;10(7):223.
Hendriks LE, Drent M, van Haren EH, Verschakelen JA, Verleden GM. Lung cancer in idiopathic pulmonary fibrosis patients diagnosed during or after lung transplantation. Respir Med Case Rep. 2012;5:37–9. https://doi.org/10.1016/j.rmedc.2011.10.003.
Daniels CE, Jett JR. Does interstitial lung disease predispose to lung cancer? Curr Opin Pulm Med. 2005;11(5):431–7. https://doi.org/10.1097/01.mcp.0000170521.71497.ba.
Tomassetti S, Gurioli C, Ryu JH, Decker PA, Ravaglia C, Tantalocco P, et al. The impact of lung cancer on survival of idiopathic pulmonary fibrosis. Chest. 2015;147(1):157–64. https://doi.org/10.1378/chest.14-0359.
Antoniou KM, Tomassetti S, Tsitoura E, Vancheri C. Idiopathic pulmonary fibrosis and lung cancer: a clinical and pathogenesis update. Curr Opin Pulm Med. 2015;21(6):626–33. https://doi.org/10.1097/mcp.0000000000000217.
Vancheri C, Failla M, Crimi N, Raghu G. Idiopathic pulmonary fibrosis: a disease with similarities and links to cancer biology. Eur Respir J. 2010;35(3):496–504. https://doi.org/10.1183/09031936.00077309.
Vancheri C. Common pathways in idiopathic pulmonary fibrosis and cancer. Eur Respir Rev. 2013;22(129):265–72. https://doi.org/10.1183/09059180.00003613.
El Agha E, Moiseenko A, Kheirollahi V, De Langhe S, Crnkovic S, Kwapiszewska G, et al. Two-way conversion between lipogenic and myogenic fibroblastic phenotypes marks the progression and resolution of lung fibrosis. Cell Stem Cell. 2017;20(2):261–73.e3. https://doi.org/10.1016/j.stem.2016.10.004.
Luo YH, Wang C, Xu WT, Zhang Y, Zhang T, Xue H, et al. 18β-glycyrrhetinic acid has anti-cancer effects via inducing apoptosis and G2/M cell cycle arrest, and inhibiting migration of A549 lung cancer cells. Onco Targets Ther. 2021;14:5131–44. https://doi.org/10.2147/ott.S322852.
Pallante P, Malapelle U, Nacchio M, Sgariglia R, Galati D, Capitelli L, et al. Liquid biopsy is a promising tool for genetic testing in idiopathic pulmonary fibrosis. Diagnostics. 2021;11(7):1202.
Jovanovic D, Roksandic Milenkovic M, Kotur Stevuljevic J, Markovic J, Ceriman V, Kontic M, et al. Membrane PD-L1 expression and soluble PD-L1 plasma levels in idiopathic pulmonary fibrosis-a pilot study. J Thorac Dis. 2018;10(12):6660–9. https://doi.org/10.21037/jtd.2018.11.16.
Marriott S, Baskir RS, Gaskill C, Menon S, Carrier EJ, Williams J, et al. ABCG2pos lung mesenchymal stem cells are a novel pericyte subpopulation that contributes to fibrotic remodeling. Am J Physiol Cell Physiol. 2014;307(8):C684–98. https://doi.org/10.1152/ajpcell.00114.2014.
Karki S, Surolia R, Hock TD, Guroji P, Zolak JS, Duggal R, et al. Wilms’ tumor 1 (Wt1) regulates pleural mesothelial cell plasticity and transition into myofibroblasts in idiopathic pulmonary fibrosis. FASEB J. 2014;28(3):1122–31. https://doi.org/10.1096/fj.13-236828.
Rock JR, Barkauskas CE, Cronce MJ, Xue Y, Harris JR, Liang J, et al. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc Natl Acad Sci U S A. 2011;108(52):E1475–83. https://doi.org/10.1073/pnas.1117988108.
Strieter RM, Keeley EC, Hughes MA, Burdick MD, Mehrad B. The role of circulating mesenchymal progenitor cells (fibrocytes) in the pathogenesis of pulmonary fibrosis. J Leuk Biol. 2009;86(5):1111–8. https://doi.org/10.1189/jlb.0309132.
Shiga K, Hara M, Nagasaki T, Sato T, Takahashi H, Takeyama H. Cancer-associated fibroblasts: their characteristics and their roles in tumor growth. Cancers (Basel). 2015;7(4):2443–58. https://doi.org/10.3390/cancers7040902.
Chen Q, Chen S, Zhao J, Zhou Y, Xu L. MicroRNA-126: a new and promising player in lung cancer. Oncol Lett. 2021;21(1):35. https://doi.org/10.3892/ol.2020.12296.
Domen A, Quatannens D, Zanivan S, Deben C, Van Audenaerde J, Smits E, et al. Cancer-associated fibroblasts as a common orchestrator of therapy resistance in lung and pancreatic cancer. Cancers (Basel). 2021;13(5) https://doi.org/10.3390/cancers13050987.
Bochet L, Lehuédé C, Dauvillier S, Wang YY, Dirat B, Laurent V, et al. Adipocyte-derived fibroblasts promote tumor progression and contribute to the desmoplastic reaction in breast cancer. Cancer Res. 2013;73(18):5657–68. https://doi.org/10.1158/0008-5472.Can-13-0530.
De P, Aske J, Dey N. Cancer-associated fibroblast functions as a road-block in cancer therapy. Cancers (Basel). 2021;13(20) https://doi.org/10.3390/cancers13205246.
Monteran L, Erez N. The dark side of fibroblasts: cancer-associated fibroblasts as mediators of immunosuppression in the tumor microenvironment. Front Immunol. 2019;1835
De Jaeghere EA, Denys HG, De Wever O. Fibroblasts fuel immune escape in the tumor microenvironment. Trends Cancer. 2019;5(11):704–23. https://doi.org/10.1016/j.trecan.2019.09.009.
Yazdani S, Bansal R, Prakash J. Drug targeting to myofibroblasts: implications for fibrosis and cancer. Adv Drug Deliv Rev. 2017;121:101–16. https://doi.org/10.1016/j.addr.2017.07.010.
Caja L, Dituri F, Mancarella S, Caballero-Diaz D, Moustakas A, Giannelli G, et al. TGF-β and the tissue microenvironment: relevance in fibrosis and cancer. Int J Mol Sci. 2018;19(5):1294.
Chen P-Y, Wei W-F, Wu H-Z, Fan L-S, Wang W. Cancer-associated fibroblast heterogeneity: a factor that cannot be ignored in immune microenvironment remodeling. Front Immunol. 2021;12:2760.
Ji X, Ji J, Shan F, Zhang Y, Chen Y, Lu X. Cancer-associated fibroblasts from NSCLC promote the radioresistance in lung cancer cell lines. Int J Clin Exp Med. 2015;8(5):7002–8.
Hua W, ten Dijke P, Kostidis S, Giera M, Hornsveld M. TGFβ-induced metabolic reprogramming during epithelial-to-mesenchymal transition in cancer. Cell Mol Life Sci. 2020;77(11):2103–23. https://doi.org/10.1007/s00018-019-03398-6.
Pereira BA, Vennin C, Papanicolaou M, Chambers CR, Herrmann D, Morton JP, et al. CAF subpopulations: a new reservoir of stromal targets in pancreatic cancer. Trends Cancer. 2019;5(11):724–41. https://doi.org/10.1016/j.trecan.2019.09.010.
Losa D, Chanson M, Crespin S. Connexins as therapeutic targets in lung disease. Expert Opin Ther Targets. 2011;15(8):989–1002. https://doi.org/10.1517/14728222.2011.584875.
Mori R, Power KT, Wang CM, Martin P, Becker DL. Acute downregulation of connexin43 at wound sites leads to a reduced inflammatory response, enhanced keratinocyte proliferation and wound fibroblast migration. J Cell Sci. 2006;119(24):5193–203. https://doi.org/10.1242/jcs.03320.
Cesen-Cummings K, Fernstrom MJ, Malkinson AM, Ruch RJ. Frequent reduction of gap junctional intercellular communication and connexin43 expression in human and mouse lung carcinoma cells. Carcinogenesis. 1998;19(1):61–7. https://doi.org/10.1093/carcin/19.1.61.
Trovato-Salinaro A, Trovato-Salinaro E, Failla M, Mastruzzo C, Tomaselli V, Gili E, et al. Altered intercellular communication in lung fibroblast cultures from patients with idiopathic pulmonary fibrosis. Respir Res. 2006;7(1):122. https://doi.org/10.1186/1465-9921-7-122.
Mazieres J, He B, You L, Xu Z, Jablons DM. Wnt signaling in lung cancer. Cancer Lett. 2005;222(1):1–10. https://doi.org/10.1016/j.canlet.2004.08.040.
Hoguin A, Rastogi A, Bowler C, Tirichine L. Genome-wide analysis of allele-specific expression of genes in the model diatom Phaeodactylum tricornutum. Sci Rep. 2021;11(1):2954. https://doi.org/10.1038/s41598-021-82529-1.
Caraci F, Gili E, Calafiore M, Failla M, La Rosa C, Crimi N, et al. TGF-beta1 targets the GSK-3beta/beta-catenin pathway via ERK activation in the transition of human lung fibroblasts into myofibroblasts. Pharmacol Res. 2008;57(4):274–82. https://doi.org/10.1016/j.phrs.2008.02.001.
Conte E, Fruciano M, Fagone E, Gili E, Caraci F, Iemmolo M, et al. Inhibition of PI3K prevents the proliferation and differentiation of human lung fibroblasts into myofibroblasts: the role of class I P110 isoforms. PLoS One. 2011;6(10):e24663.
Guerreiro AS, Fattet S, Kulesza DW, Atamer A, Elsing AN, Shalaby T, et al. A Sensitized RNA interference screen identifies a novel role for the PI3K p110γ isoform in medulloblastoma cell proliferation and chemoresistance. Mol Cancer Res. 2011;9(7):925–35. https://doi.org/10.1158/1541-7786.Mcr-10-0200.
Grimminger F, Schermuly RT, Ghofrani HA. Targeting non-malignant disorders with tyrosine kinase inhibitors. Nat Rev Drug Discov. 2010;9(12):956–70. https://doi.org/10.1038/nrd3297.
Malli F, Koutsokera A, Paraskeva E, Zakynthinos E, Papagianni M, Makris D, et al. Endothelial progenitor cells in the pathogenesis of idiopathic pulmonary fibrosis: an evolving concept. PLoS One. 2013;8(1):e53658. https://doi.org/10.1371/journal.pone.0053658.
Sakai T, Satoh K, Matsushima K, Shindo S, Abe S, Abe T, et al. Hepatocyte growth factor in bronchoalveolar lavage fluids and cells in patients with inflammatory chest diseases of the lower respiratory tract: detection by RIA and in situ hybridization. Am J Respir Cell Mol Biol. 1997;16(4):388–97. https://doi.org/10.1165/ajrcmb.16.4.9115749.
Maeda J, Ueki N, Hada T, Higashino K. Elevated serum hepatocyte growth factor/scatter factor levels in inflammatory lung disease. Am J Respir Crit Care Med. 1995;152(5):1587–91. https://doi.org/10.1164/ajrccm.152.5.7582299.
Shiratori M, Michalopoulos G, Shinozuka H, Singh G, Ogasawara H, Katyal SL. Hepatocyte growth factor stimulates DNA synthesis in alveolar epithelial type II cells in vitro. Am J Respir Cell Mol Biol. 1995;12(2):171–80. https://doi.org/10.1165/ajrcmb.12.2.7532419.
Yaekashiwa M, Nakayama S, Ohnuma K, Sakai T, Abe T, Satoh K, et al. Simultaneous or delayed administration of hepatocyte growth factor equally represses the fibrotic changes in murine lung injury induced by bleomycin. Am J Respir Crit Care Med. 1997;156(6):1937–44. https://doi.org/10.1164/ajrccm.156.6.9611057.
Panganiban RA, Day RM. Hepatocyte growth factor in lung repair and pulmonary fibrosis. Acta Pharmacol Sin. 2011;32(1):12–20. https://doi.org/10.1038/aps.2010.90.
Noth I, Zhang Y, Ma SF, Flores C, Barber M, Huang Y, et al. Genetic variants associated with idiopathic pulmonary fibrosis susceptibility and mortality: a genome-wide association study. Lancet Respir Med. 2013;1(4):309–17. https://doi.org/10.1016/s2213-2600(13)70045-6.
Nathan N, Giraud V, Picard C, Nunes H, Dastot-Le Moal F, Copin B, et al. Germline SFTPA1 mutation in familial idiopathic interstitial pneumonia and lung cancer. Hum Mol Genet. 2016;25(8):1457–67. https://doi.org/10.1093/hmg/ddw014.
Wang Y, Kuan PJ, **ng C, Cronkhite JT, Torres F, Rosenblatt RL, et al. Genetic defects in surfactant protein A2 are associated with pulmonary fibrosis and lung cancer. Am J Hum Genet. 2009;84(1):52–9. https://doi.org/10.1016/j.ajhg.2008.11.010.
Crossno PF, Polosukhin VV, Blackwell TS, Johnson JE, Markin C, Moore PE, et al. Identification of early interstitial lung disease in an individual with genetic variations in ABCA3 and SFTPC. Chest. 2010;137(4):969–73. https://doi.org/10.1378/chest.09-0790.
Stanley SE, Gable DL, Wagner CL, Carlile TM, Hanumanthu VS, Podlevsky JD, et al. Loss-of-function mutations in the RNA biogenesis factor NAF1 predispose to pulmonary fibrosis-emphysema. Sci Transl Med. 2016;8(351):351ra107. https://doi.org/10.1126/scitranslmed.aaf7837.
Kaur A, Mathai SK, Schwartz DA. Genetics in idiopathic pulmonary fibrosis pathogenesis, prognosis, and treatment. Front Med. 2017;4 https://doi.org/10.3389/fmed.2017.00154.
Nogee LM, Dunbar AE 3rd, Wert SE, Askin F, Hamvas A, Whitsett JA. A mutation in the surfactant protein C gene associated with familial interstitial lung disease. N Engl J Med. 2001;344(8):573–9. https://doi.org/10.1056/nejm200102223440805.
Thomas AQ, Lane KF, Phillips JA, Prince MA, Markin CR, Speer MC, et al. Heterozygosity for a surfactant protein C gene mutation associated with usual interstitial pneumonitis and cellular nonspecific interstitial pneumonitis in one kindred. Am J Respir Crit Care Med. 2002;165(9):1322–8.
Brasch F, Griese M, Tredano M, Johnen G, Ochs M, Rieger C, et al. Interstitial lung disease in a baby with a de novo mutation in the SFTPC gene. Eur Respir J. 2004;24(1):30–9. https://doi.org/10.1183/09031936.04.00000104.
Li J, Liepinsh E, Almlén A, Thyberg J, Curstedt T, Jörnvall H, et al. Structure and influence on stability and activity of the N-terminal propeptide part of lung surfactant protein C. FEBS J. 2006;273(5):926–35. https://doi.org/10.1111/j.1742-4658.2006.05124.x.
Maguire JA, Mulugeta S, Beers MF. Multiple ways to die: delineation of the unfolded protein response and apoptosis induced by Surfactant Protein C BRICHOS mutants. Int J Biochem Cell Biol. 2012;44(1):101–12. https://doi.org/10.1016/j.biocel.2011.10.003.
Lawson WE, Cheng D-S, Degryse AL, Tanjore H, Polosukhin VV, Xu XC, et al. Endoplasmic reticulum stress enhances fibrotic remodeling in the lungs. Proc Natl Acad Sci U S A. 2011;108(26):10562–7. https://doi.org/10.1073/pnas.1107559108.
Willander H, Hermansson E, Johansson J, Presto J. BRICHOS domain associated with lung fibrosis, dementia and cancer--a chaperone that prevents amyloid fibril formation? FEBS J. 2011;278(20):3893–904. https://doi.org/10.1111/j.1742-4658.2011.08209.x.
Tanjore H, Cheng DS, Degryse AL, Zoz DF, Abdolrasulnia R, Lawson WE, et al. Alveolar epithelial cells undergo epithelial-to-mesenchymal transition in response to endoplasmic reticulum stress. J Biol Chem. 2015;290(6):3277. https://doi.org/10.1074/jbc.A110.181164.
Zhou W, Wang Y. Candidate genes of idiopathic pulmonary fibrosis: current evidence and research. Appl Clin Genet. 2016;9:5–13. https://doi.org/10.2147/tacg.S61999.
Armanios M. Telomerase and idiopathic pulmonary fibrosis. Mutat Res. 2012;730(1–2):52–8. https://doi.org/10.1016/j.mrfmmm.2011.10.013.
Seibold MA, Wise AL, Speer MC, Steele MP, Brown KK, Loyd JE, et al. A common MUC5B promoter polymorphism and pulmonary fibrosis. N Engl J Med. 2011;364(16):1503–12. https://doi.org/10.1056/NEJMoa1013660.
Fingerlin TE, Murphy E, Zhang W, Peljto AL, Brown KK, Steele MP, et al. Genome-wide association study identifies multiple susceptibility loci for pulmonary fibrosis. Nat Genet. 2013;45(6):613–20. https://doi.org/10.1038/ng.2609.
Peljto AL, Selman M, Kim DS, Murphy E, Tucker L, Pardo A, et al. The MUC5B promoter polymorphism is associated with idiopathic pulmonary fibrosis in a Mexican cohort but is rare among Asian ancestries. Chest. 2015;147(2):460–4.
Wei R, Li C, Zhang M, Jones-Hall YL, Myers JL, Noth I, et al. Association between MUC5B and TERT polymorphisms and different interstitial lung disease phenotypes. Transl Res. 2014;163(5):494–502.
Seibold MA, Smith RW, Urbanek C, Groshong SD, Cosgrove GP, Brown KK, et al. The idiopathic pulmonary fibrosis honeycomb cyst contains a mucocilary pseudostratified epithelium. PLoS One. 2013;8(3):e58658. https://doi.org/10.1371/journal.pone.0058658.
Roy MG, Livraghi-Butrico A, Fletcher AA, McElwee MM, Evans SE, Boerner RM, et al. Muc5b is required for airway defence. Nature. 2014;505(7483):412–6. https://doi.org/10.1038/nature12807.
Yang IV, Fingerlin TE, Evans CM, Schwarz MI, Schwartz DA. MUC5B and idiopathic pulmonary fibrosis. Ann Am Thoracic Soc. 2015;12 Suppl 2(Suppl 2):S193–9. https://doi.org/10.1513/AnnalsATS.201503-110AW.
Karampitsakos T, Woolard T, Bouros D, Tzouvelekis A. Toll-like receptors in the pathogenesis of pulmonary fibrosis. Eur J Pharmacol. 2017;808:35–43. https://doi.org/10.1016/j.ejphar.2016.06.045.
Kawasaki T, Kawai T. Toll-like receptor signaling pathways. Front Immunol. 2014;5 https://doi.org/10.3389/fimmu.2014.00461.
Zhang G, Ghosh S. Negative regulation of toll-like receptor-mediated signaling by tollip*. J Biol Chem. 2002;277(9):7059–65. https://doi.org/10.1074/jbc.M109537200.
Didierlaurent A, Brissoni B, Velin D, Aebi N, Tardivel A, Käslin E, et al. Tollip regulates proinflammatory responses to interleukin-1 and lipopolysaccharide. Mol Cell Biol. 2006;26(3):735–42. https://doi.org/10.1128/MCB.26.3.735-742.2006.
Kowalski EJA, Li L. Toll-interacting protein in resolving and non-resolving inflammation. (1664–3224 (Print)).
van der Mark VA, Ghiboub M, Marsman C, Zhao J, van Dijk R, Hiralall JK, et al. Phospholipid flippases attenuate LPS-induced TLR4 signaling by mediating endocytic retrieval of Toll-like receptor 4. Cell Mol Life Sci. 2017;74(4):715–30. https://doi.org/10.1007/s00018-016-2360-5.
Trujillo G, Meneghin A, Flaherty KR, Sholl LM, Myers JL, Kazerooni EA, et al. TLR9 differentiates rapidly from slowly progressing forms of idiopathic pulmonary fibrosis. Sci Transl Med. 2010;2(57):57ra82. https://doi.org/10.1126/scitranslmed.3001510.
Korthagen NM, van Moorsel CHM, Kazemier KM, Ruven HJT, Grutters JC. IL1RN genetic variations and risk of IPF: a meta-analysis and mRNA expression study. Immunogenetics. 2012;64(5):371–7. https://doi.org/10.1007/s00251-012-0604-6.
Gasse P, Mary C, Guenon I, Noulin N, Charron S, Schnyder-Candrian S, et al. IL-1R1/MyD88 signaling and the inflammasome are essential in pulmonary inflammation and fibrosis in mice. J Clin Invest. 2007;117(12):3786–99. https://doi.org/10.1172/jci32285.
Borthwick LA. The IL-1 cytokine family and its role in inflammation and fibrosis in the lung. Semin Immunopathol. 2016;38(4):517–34. https://doi.org/10.1007/s00281-016-0559-z.
Michalski JE, Schwartz DA. Genetic risk factors for idiopathic pulmonary fibrosis: insights into immunopathogenesis. J Inflamm Res. 2020;13:1305–18. https://doi.org/10.2147/jir.S280958.
Bruder E, Hofmeister J, Aslanidis C, Hammer J, Bubendorf L, Schmitz G, et al. Ultrastructural and molecular analysis in fatal neonatal interstitial pneumonia caused by a novel ABCA3 mutation. Mod Pathol. 2007;20(10):1009–18. https://doi.org/10.1038/modpathol.3800928.
Campo I, Zorzetto M, Mariani F, Kadija Z, Morbini P, Dore R, et al. A large kindred of pulmonary fibrosis associated with a novel ABCA3 gene variant. Respir Res. 2014;15(1):43. https://doi.org/10.1186/1465-9921-15-43.
Ciantelli M, Ghirri P, Presi S, Sigali E, Vuerich M, Somaschini M, et al. Fatal respiratory failure in a full-term newborn with two ABCA3 gene mutations: a case report. J Perinatol. 2011;31(1):70–2. https://doi.org/10.1038/jp.2010.122.
Pandit KV, Milosevic J, Kaminski N. MicroRNAs in idiopathic pulmonary fibrosis. Transl Res. 2011;157(4):191–9. https://doi.org/10.1016/j.trsl.2011.01.012.
Sanders YY, Kumbla P, Hagood JS. Enhanced myofibroblastic differentiation and survival in Thy-1 (−) lung fibroblasts. Am J Respir Cell Mol Biol. 2007;36(2):226–35.
Sanders YY, Pardo A, Selman M, Nuovo GJ, Tollefsbol TO, Siegal GP, et al. Thy-1 promoter hypermethylation: a novel epigenetic pathogenic mechanism in pulmonary fibrosis. Am J Respir Cell Mol Biol. 2008;39(5):610–8.
Kuwano K, Kunitake R, Kawasaki M, Nomoto Y, Hagimoto N, Nakanishi Y, et al. P21Waf1/Cip1/Sdi1 and p53 expression in association with DNA strand breaks in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1996;154(2 Pt 1):477–83. https://doi.org/10.1164/ajrccm.154.2.8756825.
Hojo S, Fujita J, Yamadori I, Kamei T, Yoshinouchi T, Ohtsuki Y, et al. Heterogeneous point mutations of the p53 gene in pulmonary fibrosis. Eur Respir J. 1998;12(6):1404–8. https://doi.org/10.1183/09031936.98.12061404.
Uematsu K, Yoshimura A, Gemma A, Mochimaru H, Hosoya Y, Kunugi S, et al. Aberrations in the fragile histidine triad (FHIT) gene in idiopathic pulmonary fibrosis. Cancer Res. 2001;61(23):8527–33.
Demopoulos K, Arvanitis DA, Vassilakis DA, Siafakas NM, Spandidos DA. MYCL1, FHIT, SPARC, p16(INK4) and TP53 genes associated to lung cancer in idiopathic pulmonary fibrosis. J Cell Mol Med. 2002;6(2):215–22. https://doi.org/10.1111/j.1582-4934.2002.tb00188.x.
Rosales W, Carulla J, García J, Vargas D, Lizcano F. Role of histone demethylases in cardiomyocytes induced to hypertrophy. Biomed Res Int. 2016;2016:2634976. https://doi.org/10.1155/2016/2634976.
Correll KA, Edeen KE, Redente EF, Zemans RL, Edelman BL, Danhorn T, et al. TGF beta inhibits HGF, FGF7, and FGF10 expression in normal and IPF lung fibroblasts. Physiol Rep. 2018;6(16):e13794. https://doi.org/10.14814/phy2.13794.
Lovat F, Valeri N, Croce CM. MicroRNAs in the pathogenesis of Cancer. Semin Oncol. 2011;38(6):724–33. https://doi.org/10.1053/j.seminoncol.2011.08.006.
Oak SR, Murray L, Herath A, Sleeman M, Anderson I, Joshi AD, et al. A micro RNA processing defect in rapidly progressing idiopathic pulmonary fibrosis. PLoS One. 2011;6(6):e21253. https://doi.org/10.1371/journal.pone.0021253.
Corella D, Ordovas JM. Basic concepts in molecular biology related to genetics and epigenetics. Rev Española Cardiol (English Edition). 2017;70(9):744–53. https://doi.org/10.1016/j.rec.2017.05.011.
Roach KM, Feghali-Bostwick CA, Amrani Y, Bradding P. Lipoxin A4 attenuates constitutive and TGF-β1–dependent profibrotic activity in human lung myofibroblasts. J Immunol. 2015;195(6):2852–60.
Samara KD, Trachalaki A, Tsitoura E, Koutsopoulos AV, Lagoudaki ED, Lasithiotaki I, et al. Upregulation of citrullination pathway: from autoimmune to idiopathic lung fibrosis. Respir Res. 2017;18(1):218. https://doi.org/10.1186/s12931-017-0692-9.
Yang IV, Schwartz DA. Epigenetics of idiopathic pulmonary fibrosis. Transl Res. 2015;165(1):48–60. https://doi.org/10.1016/j.trsl.2014.03.011.
Yang IV. Epigenomics of idiopathic pulmonary fibrosis. (1750-192X (Electronic)).
Yang IV, Schwartz DA. Epigenetic control of gene expression in the lung. Am J Respir Crit Care Med. 2011;183(10):1295–301. https://doi.org/10.1164/rccm.201010-1579PP.
Penz-Österreicher M, Österreicher CH, Trauner M. Fibrosis in autoimmune and cholestatic liver disease. Best Pract Res Clin Gastroenterol. 2011;25(2):245–58. https://doi.org/10.1016/j.bpg.2011.02.001.
Ding Q, Luckhardt T, Hecker L, Zhou Y, Liu G, Antony VB, deAndrade J, et al. New insights into the pathogenesis and treatment of idiopathic pulmonary fibrosis. (1179–1950 (Electronic)).
Zong D, Liu X, Li J, Ouyang R, Chen P. The role of cigarette smoke-induced epigenetic alterations in inflammation. Epigenetics Chromatin. 2019;12(1):65. https://doi.org/10.1186/s13072-019-0311-8.
Pardo A, Selman M. The interplay of the genetic architecture, aging, and environmental factors in the pathogenesis of idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 2021;64(2):163–72. https://doi.org/10.1165/rcmb.2020-0373PS.
Sanders YY, Pardo A, Selman M, Nuovo GJ, Tollefsbol TO, Siegal GP, et al. Thy-1 promoter hypermethylation: a novel epigenetic pathogenic mechanism in pulmonary fibrosis. Am J Respir Cell Mol Biol. 2008;39(5):610–8. https://doi.org/10.1165/rcmb.2007-0322OC.
Hu B, Gharaee-Kermani M, Wu Z, Phan SH. Epigenetic regulation of myofibroblast differentiation by DNA methylation. (1525–2191 (Electronic)).
Tzouvelekis A, Kaminski N. Epigenetics in idiopathic pulmonary fibrosis. Biochem Cell Biol. 2015;93(2):159–70. https://doi.org/10.1139/bcb-2014-0126.
Seto E, Yoshida M. Erasers of histone acetylation: the histone deacetylase enzymes. (1943–0264 (Electronic)).
Sehgal M, Jakhete SM, Manekar AG, Sasikumar S. Specific epigenetic regulators serve as potential therapeutic targets in idiopathic pulmonary fibrosis. Heliyon. 2022;8(8):e09773. https://doi.org/10.1016/j.heliyon.2022.e09773.
Zhang M, Serna-Salas S, Damba T, Borghesan M, Demaria M, Moshage H. Hepatic stellate cell senescence in liver fibrosis: characteristics, mechanisms and perspectives. Mech Ageing Dev. 2021;199:111572. https://doi.org/10.1016/j.mad.2021.111572.
Sanders YY, Liu H, Scruggs AM, Duncan SR, Huang SK, Thannickal VJ. Epigenetic regulation of caveolin-1 gene expression in lung fibroblasts. Am J Respir Cell Mol Biol. 2017;56(1):50–61. https://doi.org/10.1165/rcmb.2016-0034OC.
Dakhlallah D, Batte K, Wang Y, Cantemir-Stone CZ, Yan P, Nuovo G, et al. Epigenetic regulation of miR-17~92 contributes to the pathogenesis of pulmonary fibrosis. Am J Respir Crit Care Med. 2013;187(4):397–405. https://doi.org/10.1164/rccm.201205-0888OC.
Lamas DJ, Kawut Smu, Bagiella E, Philip N, Arcasoy SM, Lederer DJ. Delayed access and survival in idiopathic pulmonary fibrosis: a cohort study. (1535–4970 (Electronic)).
Abuserewa ST, Duff R, Becker G. Treatment of idiopathic pulmonary fibrosis. Cureus. 2021;13(5):e15360. https://doi.org/10.7759/cureus.15360.
Noth I, Anstrom KJ, Calvert SB, de Andrade J, Flaherty KR, Glazer C, Kaner RJ, et al. A placebo-controlled randomized trial of warfarin in idiopathic pulmonary fibrosis. (1535–4970 (Electronic)).
Malouf MA, Hopkins P, Snell G, Glanville AR. An investigator-driven study of everolimus in surgical lung biopsy confirmed idiopathic pulmonary fibrosis. (1440–1843 (Electronic)).
King TE, Jr., Behr J, Brown KK, du Bois RM, Lancaster L, de Andrade JA, Stähler G, et al. BUILD-1: a randomized placebo-controlled trial of bosentan in idiopathic pulmonary fibrosis. (1535–4970 (Electronic)).
Raghu G, Behr J, Brown KK, Egan JJ, Kawut SM, Flaherty KR, Martinez FJ, et al. Treatment of idiopathic pulmonary fibrosis with ambrisentan: a parallel, randomized trial. (1539–3704 (Electronic)).
Raghu G, Million-Rousseau R, Morganti A, Perchenet L, Behr J. Macitentan for the treatment of idiopathic pulmonary fibrosis: the randomised controlled MUSIC trial. (1399–3003 (Electronic)).
Zisman Da, Schwarz M, Anstrom KJ, Collard HR, Flaherty KR, Hunninghake GW. A controlled trial of sildenafil in advanced idiopathic pulmonary fibrosis. (1533–4406 (Electronic)).
Raghu G, Brown Kk, Costabel U, Cottin V, du Bois RM, Lasky JA, Thomeer M, et al. Treatment of idiopathic pulmonary fibrosis with etanercept: an exploratory, placebo-controlled trial. (1535–4970 (Electronic)).
Daniels CE, Lasky Ja, Limper AH, Mieras K, Gabor E, Schroeder DR. Imatinib treatment for idiopathic pulmonary fibrosis: Randomized placebo-controlled trial results. (1535–4970 (Electronic)).
Helling BA, Yang IV. Epigenetics in lung fibrosis: from pathobiology to treatment perspective. Curr Opin Pulm Med. 2015;21(5):454–62. https://doi.org/10.1097/MCP.0000000000000191.
Acknowledgements
Author would like to acknowledge infrastructure facility and Research Seed Money provided by the Central University of Punjab, Bathinda, and UGC Start-Up grant from UGC-BSR and PECFAR fellowship by IGSTC. PY would like to acknowledge the SRF fellowship received from ICMR.
Conflict of Interest
None.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Singh, S.K. et al. (2023). Epigenetics of Idiopathic Pulmonary Fibrosis. In: Gupta, G., Oliver, B.G., Dua, K., Ali, M.K., Dave, P. (eds) Targeting Epigenetics in Inflammatory Lung Diseases. Springer, Singapore. https://doi.org/10.1007/978-981-99-4780-5_9
Download citation
DOI: https://doi.org/10.1007/978-981-99-4780-5_9
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-4779-9
Online ISBN: 978-981-99-4780-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)