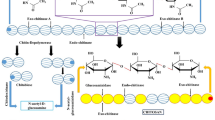
Abstract
Chitin is an insoluble structural polysaccharide present in the exoskeleton of insects. One potential biopesticide is enzyme chitinase, which degrades chitin-soluble and insoluble oligosaccharides. Recently, chitinase enzymes as a biopesticide are develo** to control insect and fungal pests—an alternative approach with enzyme-based biopesticides to avoid the chemical pesticides against pests and pathogens. The success in exploring chitinase from microbial sources, especially for agriculture, has a high volume low cost, and it also depends on the availability and active formulation of the product at a reasonable cost. The roles of chitinolytic enzymes as a biocontrol agent, different mechanisms to mass culture the chitinase and the formulations, and making the enzyme stable for the long term is the wide area to research. The chitin degradation, and identification of chitinase from different microbial sources with varying specificities, may make them more useful in commercial processes soon. In this chapter, Pseudomonas sp.-derived chitinase enzymes, essential in the agricultural field, benefit the plant by PGPR activity and mass multiplications of bacteria and the enzyme, production, and formulations in low cost, requirements to register the biopesticide and marketing were discussed in this chapter.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Arora NK (2018) Agricultural sustainability and food security. Environ Sustain 1(3):217–219
Avis TJ, Hamelin RC, Bélanger RR (2001) Approaches to molecular characterization of fungal biocontrol agents: some case studies. Can J Plant Pathol 23(1):8–12
Bahadur I, Maurya BR, Kumar A, Meena VS, Raghuwanshi R (2016) Towards the soil sustainability and potassium-solubilizing microorganisms. In: Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, pp 255–266
Barthomeuf C, Régerat F, Pourrat H (1994) Improvement in tannase recovery using the enzymatic disruption of mycelium in combination with reverse micellar enzyme extraction. Biotechnol Tech 8(2):137–142
Bhattacharya D, Nagpure A, Gupta RK (2007) Bacterial chitinases: properties and potential. Crit Rev Biotechnol 27(1):21–28
Blaak H, Schrempf H (1995) Binding and substrate specificities of a Streptomyces olivaceoviridis chitinase compared with its proteolytically processed form. Eur J Biochem 229(1):132–139
Bol JF, Linthorst HJM, Cornelissen BJC (1990) Plant pathogenesis-related proteins induced by virus infection. Annu Rev Phytopathol 28(1):113–138
Bong CFJ, Sikorowski PP (1991) Effects of cytoplasmic polyhedrosis virus and bacterial contamination on growth and development of the corn earworm, Helicoverpa zea (Lepidoptera: Noctuidae). J Invertebr Pathol 57(3):406–412
Brar SK, Verma M, Tyagi RD, Valero JR, Surampalli RY (2008) Bacillus thuringiensis fermentation of wastewater and wastewater sludge–presence and characterization of chitinases. Environ Technol 29(2):161–170
Brurberg MB, Synstad B, Klemsdal SS, van Aalten DM, Sundheim L, Eijsink VG (2001) Chitinases from Serratia marcescens. Recent Res Dev Microbiol 5:187–204
Budi SW, van Tuinen D, Arnould C, Dumas-Gaudot E, Gianinazzi-Pearson V, Gianinazzi S (2000) Hydrolytic enzyme activity of Paenibacillus sp. strain B2 and effects of the antagonistic bacterium on cell integrity of two soil-borne pathogenic fungi. Appl Soil Ecol 15(2):191–199
Cattelan AJ, Hartel PG, Fuhrmann JJ (1999) Screening for plant growth-promoting rhizobacteria to promote early soybean growth. Soil Sci Soc Am J 63(6):1670–1680
Chalidah N, Khotimah IN, Hakim AR, Meata BA, Puspita ID, Nugraheni PS, Pudjiraharti S (2018). Chitinase activity of Pseudomonas stutzeri PT5 in different fermentation conditions. In: IOP conference series: earth and environmental science, vol 139, no 1. IOP Publishing, p 012042
Chandrasekaran R, Revathi K, Nisha S, Kirubakaran SA, Sathish-Narayanan S, Senthil-Nathan S (2012) Physiological effect of chitinase purified from Bacillus subtilis against the tobacco cutworm Spodoptera litura fab. Pestic Biochem Physiol 104(1):65–71
Chavan SB, Deshpande MV (2013) Chitinolytic enzymes: an appraisal is a product of commercial potential. Biotechnol Prog 29(4):833–846
Compare RR, Nandakumar R, Kandan A, Suresh S, Bharathi M, Raguchander T, Samiyappan R (2002) Pseudomonas fluorescens based bio-formulation for the management of sheath blight disease and leaf folder insect in rice. Crop Prot 21(8):671–677
Dahiya N, Tewari R, Tiwari RP, Hoondal GS (2005) Chitinase production in solid-state fermentation by Enterobacter sp. NRG4 using statistical experimental design. Curr Microbiol 51(4):222–228
Dahiya N, Tewari R, Hoondal GS (2006) Biotechnological aspects of chitinolytic enzymes: a review. Appl Microbiol Biotechnol 71(6):773–782
David BV, Chandrasehar G, Selvam PN (2018) Pseudomonas fluorescens: a plant-growth-promoting rhizobacterium (PGPR) with potential role in biocontrol of pests of crops. In: Crop improvement through microbial biotechnology. Elsevier, pp 221–243
El-Tarabily KA, Soliman MH, Nassar AH, Al-Hassani HA, Sivasithamparam K, McKenna F, Hardy GSJ (2000) Biological control of Sclerotinia minor using a chitinolytic bacterium and actinomycetes. Plant Pathol 49(5):573–583
Faramarzi MA, Fazeli M, Yazdi MT, Adrangi S, Al-Ahmadi KJ, Tasharrofi N, Mohseni FA (2009) Optimization of cultural conditions for production of chitinase by a soil isolate of Massilia time. Biotechnology 8(1):93–99
García-Gutiérrez L, Romero D, Zeriouh H, Cazorla FM, Torés JA, de Vicente A, Pérez-García A (2012) Isolation and selection of plant growth-promoting rhizobacteria as inducers of systemic resistance in melon. Plant Soil 358(1):201–212
Gomaa EZ (2012) Chitinase production by Bacillus thuringiensis and Bacillus licheniformis: their potential in antifungal biocontrol. J Microbiol 50(1):103–111
Goswami D, Vaghela H, Parmar S, Dhandhukia P, Thakker JN (2013) Plant growth-promoting potentials of Pseudomonas spp. strain O.G. isolated from marine water. J Plant Interact 8(4):281–290
Haas D, Défago G (2005) Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat Rev Microbiol 3(4):307–319
Hamid R, Khan MA, Ahmad M, Ahmad MM, Abdin MZ, Musarrat J, Javed S (2013) Chitinases: an update. J Pharm Bioallied Sci 5(1):21
Huang CJ, Wang TK, Chung SC, Chen CY (2005) Identification of an antifungal chitinase from a potential biocontrol agent, Bacillus cereus 28-9. BMB Rep 38(1):82–88
Jain R, Pandey A (2016) A phenazine-1-carboxylic acid-producing polyextremophilic Pseudomonas chlororaphis (MCC2693) strain, isolated from mountain ecosystem, possesses biocontrol and plant growth promotion abilities. Microbiol Res 190:63–71
Khare E, Arora NK (2015) Effects of soil environment on field efficacy of microbial inoculants. In: Plant microbes symbiosis: applied facets. Springer, New Delhi, pp 353–381
Kishore GK, Pande S, Podile AR (2005) Chitin-supplemented foliar application of Serratia marcescens GPS 5 improves control of late leaf spot disease of groundnut by activating defence-related enzymes. J Phytopathol 153(3):169–173
Koga D (2004) Application of chitinase to agriculture. Biosci Ind 62:529–530
Krishnaveni S, Muthukrishnan S, Liang GH, Wilde G, Manickam A (1999) Induction of chitinases and β-1, 3-glucanases in resistant and susceptible cultivars of sorghum in response to insect attack, fungal infection and wounding. Plant Sci 144(1):9–16
Kuddus M, Ahmad IZ (2013) Isolation of novel chitinolytic bacteria and production optimization of extracellular chitinase. J Genet Eng Biotechnol 11(1):39–46
Kumar A, Bahadur I, Maurya BR, Raghuwanshi R, Meena VS, Singh DK, Dixit J (2015a) Does a plant growth-promoting rhizobacteria enhance agricultural sustainability. J Pure Appl Microbiol 9(1):715–724
Kumar V, Kumar A, Pandey KD, Roy BK (2015b) Isolation and characterization of bacterial endophytes from the roots of Cassia tora L. Ann Microbiol 65(3):1391–1399
Kumar A, Meena R, Meena VS, Bisht JK, Pattanayak A (2016) Towards the stress management and environmental sustainability. https://doi.org/10.1016/j.jclepro.2016.07.163
Lugtenberg B, Kamilova F (2009) Plant-growth-promoting rhizobacteria. Annu Rev Microbiol 63:541–556
Masood S, Bano A (2016) Mechanism of potassium solubilization in the agricultural soils with the help of soil microorganisms. In: Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, pp 137–147
Matsumoto Y, Revah S, Saucedo G, Shirai K (2001) Chitinases production in solid-state fermentation and submerged fermentation by Verticillium lecanii with silage shrimp as substrate. In: Chitin and chitosan in life sciences. Kodansha Scientific Ltd, Tokyo, pp 403–406
Matsumoto Y, Saucedo-Castañeda G, Revah S, Shirai K (2004) Production of β-N-acetylhexosaminidase of Verticillium lecanii by solid-state and submerged fermentation utilizing shrimp waste silage as substrate and inducer. Process Biochem 39(6):665–671
McHughen A, Smyth SJ (2012) Regulation of genetically modified crops in USA and Canada: American overview. In: Regulation of agricultural biotechnology: the United States and Canada. Springer, Dordrecht, pp 35–56
Meena VS, Meena SK, Bisht JK, Pattanayak A (2016) Conservation agricultural practices in sustainable food production
Miyashita K, Fujii T, Sawada Y (1991) Molecular cloning and characterization of chitinase genes from Streptomyces lividans 66. Microbiology 137(9):2065–2072
Muzzarelli RAA, Biagini G, Belmonte MM, Talassi O, Gandolfi MG, Solmi R, Carraro S, Giardino R, Fini M, Nicoli-Aldini N (1997) Osteoinduction by chitosan-complexed BMP: morpho-structural responses in an osteoporotic model. J Bioact Compat Polym 12(4):321–329
Nawaz S, Bano A (2020) Effects of PGPR (Pseudomonas sp.) and Ag-nanoparticles on enzymatic activity and physiology of cucumber. Recent Pat Food Nutr Agric 11(2):124–136
Neeraja C, Anil K, Purushotham P, Suma K, Sarma PVSRN, Moerschbacher BM, Podile AR (2010) Biotechnological approaches to develop bacterial chitinases as a bioshield against fungal diseases of plants. Crit Rev Biotechnol 30(3):231–241
Nielsen P, Sørensen J (1997) Multi-target and medium-independent fungal antagonism by hydrolytic enzymes in Paenibacillus polymyxa and Bacillus pumilus strains from barley rhizosphere. FEMS Microbiol Ecol 22(3):183–192
Oranusi NA, Trinci AP (1985) Growth of bacteria on chitin, fungal cell walls and fungal biomass, and the effect of extracellular enzymes produced by these cultures on the antifungal activity of amphotericin B. Microbios 43(172):17–30
Pathma J, Kennedy RK, Sakthivel N (2011) Mechanisms of fluorescent pseudomonads that mediate biological control of phytopathogens and plant growth promotion of crop plants. In: Bacteria in agrobiology: plant growth responses. Springer, Berlin, Heidelberg, pp 77–105
Patil RS, Ghormade V, Deshpande MV (2000) Chitinolytic enzymes: an exploration. Enzym Microb Technol 26(7):473–483
Pérez-Montaño F, Alías-Villegas C, Bellogín RA, Del Cerro P, Espuny MR, Jiménez-Guerrero I, López-Baena FJ, Ollero FJ, Cubo T (2014) Plant growth promotion in cereal and leguminous agricultural important plants: from microorganism capacities to crop production. Microbiol Res 169(5-6):325–336
Poulsen PH, Møller J, Magid J (2008) Determination of a relationship between chitinase activity and microbial diversity in chitin amended compost. Bioresour Technol 99(10):4355–4359
Quesssaoui R, Bouharroud R, Amarraque A, Ajerrar A, Mayad EH, Chebli B, Dadi M, Elaini R, El Filali F, Walters AS (2017) Ecological applications of Pseudomonas as a biopesticide to control two-spotted mite Tetranychus urticae: chitinase and HCN production. J Plant Protect Res 57(4)
Rathore AS, Gupta RD (2015) Chitinases from bacteria to human: properties, applications, and future perspectives. Enzyme Res 2015:791907
Rishad KS, Rebello S, Nathan VK, Shabanamol S, Jisha MS (2016) Optimised production of chitinase from a novel mangrove isolate, Bacillus pumilus MCB-7 using response surface methodology. Biocatal Agric Biotechnol 5:143–149
Robert N, Roche K, Lebeau Y, Breda C, Boulay M, Esnault R, Buffard D (2002) Expression of grapevine chitinase genes in berries and leaves infected by fungal or bacterial pathogens. Plant Sci 162(3):389–400
Shirai K, Guerrero I, Huerta S, Saucedo G, Castillo A, Gonzalez RO, Hall GM (2001) Effect of initial glucose concentration and inoculation level of lactic acid bacteria in shrimp waste ensilation. Enzym Microb Technol 28(4-5):446–452
Sindhu SS, Dadarwal KR (2001) Chitinolytic and cellulolytic Pseudomonas sp. antagonistic to fungal pathogens enhances nodulation by Mesorhizobium sp. Cicer in chickpea. Microbiol Res 156(4):353–358
Singh PP, Shin YC, Park CS, Chung YR (1999) Biological control of Fusarium wilt of cucumber by chitinolytic bacteria. Phytopathology 89(1):92–99
Smirnoff WA (1974) Three years of aerial field experiments with Bacillus thuringiensis plus chitinase formulation against the spruce budworm. J Invertebr Pathol 24(3):344–348
Someya N, Kataoka N, Komagata T, Hirayae K, Hibi T, Akutsu K (2000) Biological control of cyclamen soil-borne diseases by Serratia marcescens strain B2. Plant Dis 84(3):334–340
Synstad B, Vaaje-Kolstad G, Cederkvist FH, Saua SF, Horn SJ, Eijsink VG, Sørlie M (2008) Expression and characterization of endochitinase C from Serratia marcescens BJL200 and its purification by a one-step general chitinase purification method. Biosci Biotechnol Biochem 72(3):715–723
Takayanagi T, Ajisaka K, Takiguchi Y, Shimahara K (1991) Isolation and characterization of thermostable chitinases from Bacillus licheniformis X-7u. Biochim Biophys Acta 1078(3):404–410
Tanaka H, Ogasawara N, Nakajima T, Tamari K (1970) Cell walls of Pirigularia oryzae I. Selective enzymolysis of Pirigularia oryzae walls by wall-lytic enzymes of Bacillus circulans WL 12. J Gen Appl Microbiol 16(1):39–60
Teotia P, Kumar V, Kumar M, Shrivastava N, Varma A (2016) Rhizosphere microbes: potassium solubilization and crop productivity–present and future aspects. In: Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, pp 315–325
Van Loon LC, Bakker PAHM, Pieterse CMJ (1998) Systemic resistance induced by rhizosphere bacteria. Annu Rev Phytopathol 36(1):453–483
Vega K, Kalkum M (2012) Chitin, chitinase responses, and invasive fungal infections. Int J Microbiol 2012:920459
Wang SL, Chang WT (1997) Purification and characterization of two bifunctional chitinases/lysozymes extracellularly produced by Pseudomonas aeruginosa K-187 in a shrimp and crab shell powder medium. Appl Environ Microbiol 63(2):380–386
Wang SL, Chang WT, Lu MC (1995) Production of chitinase by Pseudomonas aeruginosa K-187 using shrimp and crab shell powder as a carbon source. Proc Natl Sci Counc Repub China B 19(2):105–112
Wang SL, Shih IL, Liang TW, Wang CH (2002) Purification and characterization of two antifungal chitinases extracellularly produced by Bacillus amyloliquefaciens V656 in a shrimp and crab shell powder medium. J Agric Food Chem 50(8):2241–2248
Wang SL, Lin TY, Yen YH, Liao HF, Chen YJ (2006) Bioconversion of shellfish chitin wastes for the production of Bacillus subtilis W-118 chitinase. Carbohydr Res 341(15):2507–2515
Wang SL, Chao CH, Liang TW, Chen CC (2009) Purification and characterization of protease and chitinase from Bacillus cereus TKU006 and conversion of marine wastes by these enzymes. Mar Biotechnol 11(3):334–344
Wang SL, Lin BS, Liang TW, Wang CL, Wu PC, Liu JR (2010) Purification and characterization of chitinase from a new species strain, Pseudomonas sp. TKU008. J Microbiol Biotechnol 20(6):1001–1005
Yasir M, Aslam Z, Kim SW, Lee SW, Jeon CO, Chung YR (2009) Bacterial community composition and chitinase gene diversity of vermicompost with antifungal activity. Bioresour Technol 100(19):4396–4403
Acknowledgments
The authors thank Society for Research and Initiatives for Sustainable Technologies and Institutions, Grambharti, Gujarat-382735, India, for providing the necessary facilities for writing this book chapter.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Vivekanandhan, P., Swathy, K., Chordia, M.A. (2022). Pseudomonas Species-Derived Chitinase Mass Multiplication, Production Cost Analysis, and Marketing: As a Biocontrol Agent for Crop Protection. In: Amaresan, N., Dharumadurai, D., Cundell, D.R. (eds) Industrial Microbiology Based Entrepreneurship. Microorganisms for Sustainability, vol 42. Springer, Singapore. https://doi.org/10.1007/978-981-19-6664-4_12
Download citation
DOI: https://doi.org/10.1007/978-981-19-6664-4_12
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-6663-7
Online ISBN: 978-981-19-6664-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)