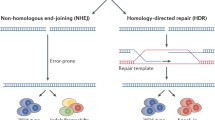
Abstract
Differential gene expression is associated with diabetic cardiomyopathy (DMCM) and culminates in adverse remodeling in the diabetic heart. Genome editing is a technology utilized to alter endogenous genes. Genome editing also provides an option to induce cardioprotective genes or inhibit genes linked to adverse cardiac remodeling and thus has promise in ameliorating DMCM. Non-coding genes have emerged as novel regulators of cellular signaling and may serve as potential therapeutic targets for DMCM. Specifically, there is a widespread change in the gene expression of fetal cardiac genes and microRNAs, termed genetic reprogramming, that promotes pathological remodeling and contributes to heart failure in diabetes. This genetic reprogramming of both coding and non-coding genes varies with the progression and severity of DMCM. Thus, genetic editing provides a promising option to investigate the role of specific genes/non-coding RNAs in DMCM initiation and progression as well as develo** therapeutics to mitigate cardiac remodeling and ameliorate DMCM. This chapter will summarize the research progress in genome editing and DMCM and provide future directions for utilizing genome editing as an approach to prevent and/or treat DMCM.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, Grishman A (1972) New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol 30(6):595–602
Boudina S, Abel ED (2010) Diabetic cardiomyopathy, causes and effects. Rev Endocr Metab Disord 11(1):31–39
Chavali V, Tyagi SC, Mishra PK (2013) Predictors and prevention of diabetic cardiomyopathy. Diabetes Metab Syndr Obes 6:151–160
Vinik AI, Ziegler D (2007) Diabetic cardiovascular autonomic neuropathy. Circulation 115(3):387–397
Pop-Busui R (2010) Cardiac autonomic neuropathy in diabetes: a clinical perspective. Diabetes Care 33(2):434–441
Braunwald E (2019) Diabetes, heart failure, and renal dysfunction: the vicious circles. Prog Cardiovasc Dis 62(4):298–302
Aguilar D (2016) Heart failure, diabetes mellitus, and chronic kidney disease: a clinical conundrum. Circ Heart Fail 9(7)
Pervolaraki E, Dachtler J, Anderson RA, Holden AV (2018) The developmental transcriptome of the human heart. Sci Rep 8(1):15362
Thum T, Galuppo P, Wolf C, Fiedler J, Kneitz S, van Laake LW, Doevendans PA, Mummery CL, Borlak J, Haverich A, Gross C, Engelhardt S, Ertl G, Bauersachs J (2007) MicroRNAs in the human heart: a clue to fetal gene reprogramming in heart failure. Circulation 116(3):258–267
Ramirez Flores RO, Lanzer JD, Holland CH, Leuschner F, Most P, Schultz JH, Levinson RT, Saez-Rodriguez J (2021) Consensus transcriptional landscape of human end-stage heart failure. J Am Heart Assoc 10(7):e019667
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116(2):281–297
Bartel DP (2009) MicroRNAs: target recognition and regulatory functions. Cell 136(2):215–233
Gholaminejad A, Zare N, Dana N, Shafie D, Mani A, Javanmard SH (2021) A meta-analysis of microRNA expression profiling studies in heart failure. Heart Fail Rev 26(4):997–1021
Hall B, Limaye A, Kulkarni AB (2009) Overview: generation of gene knockout mice. Curr Protoc Cell Biol Chapter 19:Unit 19.12–19.12.1-17
Matkovich SJ, Wang W, Tu Y, Eschenbacher WH, Dorn LE, Condorelli G, Diwan A, Nerbonne JM, Dorn GW 2nd (2010) MicroRNA-133a protects against myocardial fibrosis and modulates electrical repolarization without affecting hypertrophy in pressure-overloaded adult hearts. Circ Res 106(1):166–175
Fernandez-Chacon M, Casquero-Garcia V, Luo W, Francesca Lunella F, Ferreira Rocha S, Del Olmo-Cabrera S, Benedito R (2019) iSuRe-Cre is a genetic tool to reliably induce and report Cre-dependent genetic modifications. Nat Commun 10(1):2262
Kesherwani V, Shahshahan HR, Mishra PK (2017) Cardiac transcriptome profiling of diabetic Akita mice using microarray and next generation sequencing. PLoS One 12(8):e0182828
Muller HJ (1927) Artificial transmutation of the gene. Science 66(1699):84–87
Auerbach C, Robson JM, Carr JG (1947) The chemical production of mutations. Science 105(2723):243–247
Scherer S, Davis RW (1979) Replacement of chromosome segments with altered DNA sequences constructed in vitro. Proc Natl Acad Sci U S A 76(10):4951–4955
Rothstein RJ (1983) One-step gene disruption in yeast. Methods Enzymol 101:202–211
Smithies O, Gregg RG, Boggs SS, Koralewski MA, Kucherlapati RS (1985) Insertion of DNA sequences into the human chromosomal beta-globin locus by homologous recombination. Nature 317(6034):230–234
Thomas KR, Folger KR, Capecchi MR (1986) High frequency targeting of genes to specific sites in the mammalian genome. Cell 44(3):419–428
Mansour SL, Thomas KR, Capecchi MR (1988) Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature 336(6197):348–352
Youds JL, Boulton SJ (2011) The choice in meiosis—defining the factors that influence crossover or non-crossover formation. J Cell Sci 124(Pt 4):501–513
Carroll D (2014) Genome engineering with targetable nucleases. Annu Rev Biochem 83:409–439
Choulika A, Perrin A, Dujon B, Nicolas JF (1995) Induction of homologous recombination in mammalian chromosomes by using the I-SceI system of Saccharomyces cerevisiae. Mol Cell Biol 15(4):1968–1973
Plessis A, Perrin A, Haber JE, Dujon B (1992) Site-specific recombination determined by I-SceI, a mitochondrial group I intron-encoded endonuclease expressed in the yeast nucleus. Genetics 130(3):451–460
Rouet P, Smih F, Jasin M (1994) Introduction of double-strand breaks into the genome of mouse cells by expression of a rare-cutting endonuclease. Mol Cell Biol 14(12):8096–8106
Rudin N, Sugarman E, Haber JE (1989) Genetic and physical analysis of double-strand break repair and recombination in Saccharomyces cerevisiae. Genetics 122(3):519–534
Chapman JR, Taylor MR, Boulton SJ (2012) Playing the end game: DNA double-strand break repair pathway choice. Mol Cell 47(4):497–510
Randhawa S, Sengar S (2021) The evolution and history of gene editing technologies. Prog Mol Biol Transl Sci 178:1–62
Gaj T, Sirk SJ, Shui SL, Liu J (2016) Genome-editing technologies: principles and applications. Cold Spring Harb Perspect Biol 8(12):a023754
Gaj T, Gersbach CA, Barbas CF 3rd (2013) ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol 31(7):397–405
Khan SH (2019) Genome-editing technologies: concept, pros, and cons of various genome-editing techniques and bioethical concerns for clinical application. Mol Ther Nucleic Acids 16:326–334
Urnov FD, Rebar EJ, Holmes MC, Zhang HS, Gregory PD (2010) Genome editing with engineered zinc finger nucleases. Nat Rev Genet 11(9):636–646
Carroll D (2008) Progress and prospects: zinc-finger nucleases as gene therapy agents. Gene Ther 15(22):1463–1468
Miller J, McLachlan AD, Klug A (1985) Repetitive zinc-binding domains in the protein transcription factor IIIA from Xenopus oocytes. EMBO J 4(6):1609–1614
Kim YG, Cha J, Chandrasegaran S (1996) Hybrid restriction enzymes: zinc finger fusions to Fok I cleavage domain. Proc Natl Acad Sci U S A 93(3):1156–1160
Porteus MH, Carroll D (2005) Gene targeting using zinc finger nucleases. Nat Biotechnol 23(8):967–973
Smith J, Bibikova M, Whitby FG, Reddy AR, Chandrasegaran S, Carroll D (2000) Requirements for double-strand cleavage by chimeric restriction enzymes with zinc finger DNA-recognition domains. Nucleic Acids Res 28(17):3361–3369
Pavletich NP, Pabo CO (1991) Zinc finger-DNA recognition: crystal structure of a Zif268-DNA complex at 2.1 A. Science 252(5007):809–817
Segal DJ, Dreier B, Beerli RR, Barbas CF 3rd (1999) Toward controlling gene expression at will: selection and design of zinc finger domains recognizing each of the 5′-GNN-3′ DNA target sequences. Proc Natl Acad Sci U S A 96(6):2758–2763
Dreier B, Beerli RR, Segal DJ, Flippin JD, Barbas CF 3rd (2001) Development of zinc finger domains for recognition of the 5′-ANN-3′ family of DNA sequences and their use in the construction of artificial transcription factors. J Biol Chem 276(31):29466–29478
Bae KH, Kwon YD, Shin HC, Hwang MS, Ryu EH, Park KS, Yang HY, Lee DK, Lee Y, Park J, Kwon HS, Kim HW, Yeh BI, Lee HW, Sohn SH, Yoon J, Seol W, Kim JS (2003) Human zinc fingers as building blocks in the construction of artificial transcription factors. Nat Biotechnol 21(3):275–280
Liu Q, Segal DJ, Ghiara JB, Barbas CF 3rd (1997) Design of polydactyl zinc-finger proteins for unique addressing within complex genomes. Proc Natl Acad Sci U S A 94(11):5525–5530
Beerli RR, Segal DJ, Dreier B, Barbas CF 3rd (1998) Toward controlling gene expression at will: specific regulation of the erbB-2/HER-2 promoter by using polydactyl zinc finger proteins constructed from modular building blocks. Proc Natl Acad Sci U S A 95(25):14628–14633
Beerli RR, Dreier B, Barbas CF 3rd (2000) Positive and negative regulation of endogenous genes by designed transcription factors. Proc Natl Acad Sci U S A 97(4):1495–1500
Kim HJ, Lee HJ, Kim H, Cho SW, Kim JS (2009) Targeted genome editing in human cells with zinc finger nucleases constructed via modular assembly. Genome Res 19(7):1279–1288
Greisman HA, Pabo CO (1997) A general strategy for selecting high-affinity zinc finger proteins for diverse DNA target sites. Science 275(5300):657–661
Isalan M, Klug A, Choo Y (2001) A rapid, generally applicable method to engineer zinc fingers illustrated by targeting the HIV-1 promoter. Nat Biotechnol 19(7):656–660
Hurt JA, Thibodeau SA, Hirsh AS, Pabo CO, Joung JK (2003) Highly specific zinc finger proteins obtained by directed domain shuffling and cell-based selection. Proc Natl Acad Sci U S A 100(21):12271–12276
Magnenat L, Schwimmer LJ, Barbas CF 3rd (2008) Drug-inducible and simultaneous regulation of endogenous genes by single-chain nuclear receptor-based zinc-finger transcription factor gene switches. Gene Ther 15(17):1223–1232
Maeder ML, Thibodeau-Beganny S, Osiak A, Wright DA, Anthony RM, Eichtinger M, Jiang T, Foley JE, Winfrey RJ, Townsend JA, Unger-Wallace E, Sander JD, Muller-Lerch F, Fu F, Pearlberg J, Gobel C, Dassie JP, Pruett-Miller SM, Porteus MH, Sgroi DC, Iafrate AJ, Dobbs D, McCray PB Jr, Cathomen T, Voytas DF, Joung JK (2008) Rapid “open-source” engineering of customized zinc-finger nucleases for highly efficient gene modification. Mol Cell 31(2):294–301
Bhakta MS, Henry IM, Ousterout DG, Das KT, Lockwood SH, Meckler JF, Wallen MC, Zykovich A, Yu Y, Leo H, Xu L, Gersbach CA, Segal DJ (2013) Highly active zinc-finger nucleases by extended modular assembly. Genome Res 23(3):530–538
Gupta A, Christensen RG, Rayla AL, Lakshmanan A, Stormo GD, Wolfe SA (2012) An optimized two-finger archive for ZFN-mediated gene targeting. Nat Methods 9(6):588–590
Sander JD, Dahlborg EJ, Goodwin MJ, Cade L, Zhang F, Cifuentes D, Curtin SJ, Blackburn JS, Thibodeau-Beganny S, Qi Y, Pierick CJ, Hoffman E, Maeder ML, Khayter C, Reyon D, Dobbs D, Langenau DM, Stupar RM, Giraldez AJ, Voytas DF, Peterson RT, Yeh JR, Joung JK (2011) Selection-free zinc-finger-nuclease engineering by context-dependent assembly (CoDA). Nat Methods 8(1):67–69
Boch J, Scholze H, Schornack S, Landgraf A, Hahn S, Kay S, Lahaye T, Nickstadt A, Bonas U (2009) Breaking the code of DNA binding specificity of TAL-type III effectors. Science 326(5959):1509–1512
Hensel G, Kumlehn J (2019) Genome engineering using TALENs. Methods Mol Biol 1900:195–215
Naert T, Van Nieuwenhuysen T, Vleminckx K (2017) TALENs and CRISPR/Cas9 fuel genetically engineered clinically relevant Xenopus tropicalis tumor models. Genesis 55(1–2)
Martin-Fernandez JM, Fleischer A, Vallejo-Diez S, Palomino E, Sanchez-Gilabert A, Ruiz R, Bejarano Y, Llinas P, Gaya A, Bachiller D (2020) New bicistronic TALENs greatly improve genome editing. Curr Protoc Stem Cell Biol 52(1):e104
Fang Y, Cheng Y, Lu D, Gong X, Yang G, Gong Z, Zhu Y, Sang X, Fan S, Zhang J, Zeng F (2018) Treatment of beta(654)-thalassaemia by TALENs in a mouse model. Cell Prolif 51(6):e12491
Bi H, Fei Q, Li R, Liu B, **a R, Char SN, Meyers BC, Yang B (2020) Disruption of miRNA sequences by TALENs and CRISPR/Cas9 induces varied lengths of miRNA production. Plant Biotechnol J 18(7):1526–1536
Sun N, Zhao H (2013) Transcription activator-like effector nucleases (TALENs): a highly efficient and versatile tool for genome editing. Biotechnol Bioeng 110(7):1811–1821
Liu Y, Zhao H, Cheng CH (2016) Mutagenesis in Xenopus and zebrafish using TALENs. Methods Mol Biol 1338:207–227
Joung JK, Sander JD (2013) TALENs: a widely applicable technology for targeted genome editing. Nat Rev Mol Cell Biol 14(1):49–55
Chen SJ, Chen YC (2019) Potential application of TALENs against murine cytomegalovirus latent infections. Viruses 11(5):414
Chen K, Gao C (2013) TALENs: customizable molecular DNA scissors for genome engineering of plants. J Genet Genomics 40(6):271–279
Luo D, Feng K, Zhu Z, Hu W (2019) Generating gene knockout Oryzias latipes and Rice field eel using TALENs method. Methods Mol Biol 1874:489–506
Qasim W, Zhan H, Samarasinghe S, Adams S, Amrolia P, Stafford S, Butler K, Rivat C, Wright G, Somana K, Ghorashian S, Pinner D, Ahsan G, Gilmour K, Lucchini G, Inglott S, Mifsud W, Chiesa R, Peggs KS, Chan L, Farzeneh F, Thrasher AJ, Vora A, Pule M, Veys P (2017) Molecular remission of infant B-ALL after infusion of universal TALEN gene-edited CAR T cells. Sci Transl Med 9(374):eaaj2013
Yu AQ, Ding Y, Lu ZY, Hao YZ, Teng ZP, Yan SR, Li DS, Zeng Y (2018) TALENs-mediated homozygous CCR5Delta32 mutations endow CD4+ U87 cells with resistance against HIV1 infection. Mol Med Rep 17(1):243–249
Kazama T, Okuno M, Watari Y, Yanase S, Koizuka C, Tsuruta Y, Sugaya H, Toyoda A, Itoh T, Tsutsumi N, Toriyama K, Koizuka N, Arimura SI (2019) Curing cytoplasmic male sterility via TALEN-mediated mitochondrial genome editing. Nat Plants 5(7):722–730
Wang Y, Tan A, Xu J, Li Z, Zeng B, Ling L, You L, Chen Y, James AA, Huang Y (2014) Site-specific, TALENs-mediated transformation of Bombyx mori. Insect Biochem Mol Biol 55:26–30
Feng Y, Zhang S, Huang X (2014) A robust TALENs system for highly efficient mammalian genome editing. Sci Rep 4:3632
Gan Y, Lin Y, Guo Y, Qi X, Wang Q (2018) Metabolic and genomic characterisation of stress-tolerant industrial Saccharomyces cerevisiae strains from TALENs-assisted multiplex editing. FEMS Yeast Res 18(5)
Zhang G, Lin Y, Qi X, Li L, Wang Q, Ma Y (2015) TALENs-assisted multiplex editing for accelerated genome evolution to improve yeast phenotypes. ACS Synth Biol 4(10):1101–1111
**a E, Zhang Y, Cao H, Li J, Duan R, Hu J (2019) TALEN-mediated gene targeting for cystic fibrosis-gene therapy. Genes (Basel) 10(1):39
Sun N, Zhao H (2014) Seamless correction of the sickle cell disease mutation of the HBB gene in human induced pluripotent stem cells using TALENs. Biotechnol Bioeng 111(5):1048–1053
Bacman SR, Kauppila JHK, Pereira CV, Nissanka N, Miranda M, Pinto M, Williams SL, Larsson NG, Stewart JB, Moraes CT (2018) MitoTALEN reduces mutant mtDNA load and restores tRNA(ala) levels in a mouse model of heteroplasmic mtDNA mutation. Nat Med 24(11):1696–1700
Meng D, Han S, Jeong IS, Kim SW (2019) Interleukin 10-secreting MSCs via TALEN-mediated gene editing attenuates left ventricular remodeling after myocardial infarction. Cell Physiol Biochem 52(4):728–741
Baker M (2012) Gene-editing nucleases. Nat Methods 9(1):23–26
Carroll D (2017) Genome editing: past, present, and future. Yale J Biol Med 90(4):653–659
Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F (2013) Genome engineering using the CRISPR-Cas9 system. Nat Protoc 8(11):2281–2308
Ma Y, Zhang L, Huang X (2014) Genome modification by CRISPR/Cas9. FEBS J 281(23):5186–5193
Redman M, King A, Watson C, King D (2016) What is CRISPR/Cas9? Arch Dis Child Educ Pract Ed 101(4):213–215
Wiedenheft B, Sternberg SH, Doudna JA (2012) RNA-guided genetic silencing systems in bacteria and archaea. Nature 482(7385):331–338
Sorek R, Lawrence CM, Wiedenheft B (2013) CRISPR-mediated adaptive immune systems in bacteria and archaea. Annu Rev Biochem 82:237–266
Hryhorowicz M, Lipinski D, Zeyland J, Slomski R (2017) CRISPR/Cas9 immune system as a tool for genome engineering. Arch Immunol Ther Exp (Warsz) 65(3):233–240
Horvath P, Barrangou R (2010) CRISPR/Cas, the immune system of bacteria and archaea. Science 327(5962):167–170
Marraffini LA, Sontheimer EJ (2010) CRISPR interference: RNA-directed adaptive immunity in bacteria and archaea. Nat Rev Genet 11(3):181–190
**ek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E (2012) A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337(6096):816–821
Ren X, Sun J, Housden BE, Hu Y, Roesel C, Lin S, Liu LP, Yang Z, Mao D, Sun L, Wu Q, Ji JY, ** J, Mohr SE, Xu J, Perrimon N, Ni JQ (2013) Optimized gene editing technology for Drosophila melanogaster using germ line-specific Cas9. Proc Natl Acad Sci U S A 110(47):19012–19017
Cho SW, Kim S, Kim JM, Kim JS (2013) Targeted genome engineering in human cells with the Cas9 RNA-guided endonuclease. Nat Biotechnol 31(3):230–232
Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, Zhang F (2013) Multiplex genome engineering using CRISPR/Cas systems. Science 339(6121):819–823
Mali P, Yang L, Esvelt KM, Aach J, Guell M, DiCarlo JE, Norville JE, Church GM (2013) RNA-guided human genome engineering via Cas9. Science 339(6121):823–826
Nishimasu H, Ran FA, Hsu PD, Konermann S, Shehata SI, Dohmae N, Ishitani R, Zhang F, Nureki O (2014) Crystal structure of Cas9 in complex with guide RNA and target DNA. Cell 156(5):935–949
Anders C, Niewoehner O, Duerst A, **ek M (2014) Structural basis of PAM-dependent target DNA recognition by the Cas9 endonuclease. Nature 513(7519):569–573
Jiang F, Taylor DW, Chen JS, Kornfeld JE, Zhou K, Thompson AJ, Nogales E, Doudna JA (2016) Structures of a CRISPR-Cas9 R-loop complex primed for DNA cleavage. Science 351(6275):867–871
Cradick TJ, Fine EJ, Antico CJ, Bao G (2013) CRISPR/Cas9 systems targeting beta-globin and CCR5 genes have substantial off-target activity. Nucleic Acids Res 41(20):9584–9592
Fu Y, Foden JA, Khayter C, Maeder ML, Reyon D, Joung JK, Sander JD (2013) High-frequency off-target mutagenesis induced by CRISPR-Cas nucleases in human cells. Nat Biotechnol 31(9):822–826
Mali P, Aach J, Stranges PB, Esvelt KM, Moosburner M, Kosuri S, Yang L, Church GM (2013) CAS9 transcriptional activators for target specificity screening and paired nickases for cooperative genome engineering. Nat Biotechnol 31(9):833–838
Ran FA, Hsu PD, Lin CY, Gootenberg JS, Konermann S, Trevino AE, Scott DA, Inoue A, Matoba S, Zhang Y, Zhang F (2013) Double nicking by RNA-guided CRISPR Cas9 for enhanced genome editing specificity. Cell 154(6):1380–1389
Fu Y, Sander JD, Reyon D, Cascio VM, Joung JK (2014) Improving CRISPR-Cas nuclease specificity using truncated guide RNAs. Nat Biotechnol 32(3):279–284
Hsu PD, Scott DA, Weinstein JA, Ran FA, Konermann S, Agarwala V, Li Y, Fine EJ, Wu X, Shalem O, Cradick TJ, Marraffini LA, Bao G, Zhang F (2013) DNA targeting specificity of RNA-guided Cas9 nucleases. Nat Biotechnol 31(9):827–832
Zetsche B, Volz SE, Zhang F (2015) A split-Cas9 architecture for inducible genome editing and transcription modulation. Nat Biotechnol 33(2):139–142
Davis KM, Pattanayak V, Thompson DB, Zuris JA, Liu DR (2015) Small molecule-triggered Cas9 protein with improved genome-editing specificity. Nat Chem Biol 11(5):316–318
Savic N, Schwank G (2016) Advances in therapeutic CRISPR/Cas9 genome editing. Transl Res 168:15–21
Gupta D, Bhattacharjee O, Mandal D, Sen MK, Dey D, Dasgupta A, Kazi TA, Gupta R, Sinharoy S, Acharya K, Chattopadhyay D, Ravichandiran V, Roy S, Ghosh D (2019) CRISPR-Cas9 system: a new-fangled dawn in gene editing. Life Sci 232:116636
Cai L, Fisher AL, Huang H, **e Z (2016) CRISPR-mediated genome editing and human diseases. Genes Dis 3(4):244–251
Chen M, Mao A, Xu M, Weng Q, Mao J, Ji J (2019) CRISPR-Cas9 for cancer therapy: opportunities and challenges. Cancer Lett 447:48–55
Min YL, Li H, Rodriguez-Caycedo C, Mireault AA, Huang J, Shelton JM, McAnally JR, Amoasii L, Mammen PPA, Bassel-Duby R, Olson EN (2019) CRISPR-Cas9 corrects Duchenne muscular dystrophy exon 44 deletion mutations in mice and human cells. Sci Adv 5(3):eaav4324
Bengtsson NE, Hall JK, Odom GL, Phelps MP, Andrus CR, Hawkins RD, Hauschka SD, Chamberlain JR, Chamberlain JS (2017) Muscle-specific CRISPR/Cas9 dystrophin gene editing ameliorates pathophysiology in a mouse model for Duchenne muscular dystrophy. Nat Commun 8:14454
Demirci S, Leonard A, Haro-Mora JJ, Uchida N, Tisdale JF (2019) CRISPR/Cas9 for sickle cell disease: applications, future possibilities, and challenges. Adv Exp Med Biol 1144:37–52
Tessadori F, Roessler HI, Savelberg SMC, Chocron S, Kamel SM, Duran KJ, van Haelst MM, van Haaften G, Bakkers J (2018) Effective CRISPR/Cas9-based nucleotide editing in zebrafish to model human genetic cardiovascular disorders. Dis Model Mech 11(10):dmm035469
Liu X, Yagi H, Saeed S, Bais AS, Gabriel GC, Chen Z, Peterson KA, Li Y, Schwartz MC, Reynolds WT, Saydmohammed M, Gibbs B, Wu Y, Devine W, Chatterjee B, Klena NT, Kostka D, de Mesy Bentley KL, Ganapathiraju MK, Dexheimer P, Leatherbury L, Khalifa O, Bhagat A, Zahid M, Pu W, Watkins S, Grossfeld P, Murray SA, Porter GA Jr, Tsang M, Martin LJ, Benson DW, Aronow BJ, Lo CW (2017) The complex genetics of hypoplastic left heart syndrome. Nat Genet 49(7):1152–1159
**e C, Zhang YP, Song L, Luo J, Qi W, Hu J, Lu D, Yang Z, Zhang J, **ao J, Zhou B, Du JL, **g N, Liu Y, Wang Y, Li BL, Song BL, Yan Y (2016) Genome editing with CRISPR/Cas9 in postnatal mice corrects PRKAG2 cardiac syndrome. Cell Res 26(10):1099–1111
Sano S, Oshima K, Wang Y, Katanasaka Y, Sano M, Walsh K (2018) CRISPR-mediated gene editing to assess the roles of Tet2 and Dnmt3a in clonal hematopoiesis and cardiovascular disease. Circ Res 123(3):335–341
Schoger E, Carroll KJ, Iyer LM, McAnally JR, Tan W, Liu N, Noack C, Shomroni O, Salinas G, Gross J, Herzog N, Doroudgar S, Bassel-Duby R, Zimmermann WH, Zelarayan LC (2020) CRISPR-mediated activation of endogenous gene expression in the postnatal heart. Circ Res 126(1):6–24
Mosqueira D, Mannhardt I, Bhagwan JR, Lis-Slimak K, Katili P, Scott E, Hassan M, Prondzynski M, Harmer SC, Tinker A, Smith JGW, Carrier L, Williams PM, Gaffney D, Eschenhagen T, Hansen A, Denning C (2018) CRISPR/Cas9 editing in human pluripotent stem cell-cardiomyocytes highlights arrhythmias, hypocontractility, and energy depletion as potential therapeutic targets for hypertrophic cardiomyopathy. Eur Heart J 39(43):3879–3892
Vermersch E, Jouve C, Hulot JS (2020) CRISPR/Cas9 gene-editing strategies in cardiovascular cells. Cardiovasc Res 116(5):894–907
Mishra PK, Tyagi N, Sen U, Joshua IG, Tyagi SC (2010) Synergism in hyperhomocysteinemia and diabetes: role of PPAR gamma and tempol. Cardiovasc Diabetol 9:49
Chavali V, Nandi SS, Singh SR, Mishra PK (2014) Generating double knockout mice to model genetic intervention for diabetic cardiomyopathy in humans. Methods Mol Biol 1194:385–400
Prathipati P, Metreveli N, Nandi SS, Tyagi SC, Mishra PK (2016) Ablation of matrix Metalloproteinase-9 prevents cardiomyocytes contractile dysfunction in diabetics. Front Physiol 7:93
Kumarswamy R, Thum T (2013) Non-coding RNAs in cardiac remodeling and heart failure. Circ Res 113(6):676–689
Leptidis S, El Azzouzi H, Lok SI, de Weger R, Olieslagers S, Kisters N, Silva GJ, Heymans S, Cuppen E, Berezikov E, De Windt LJ, da Costa MP (2013) A deep sequencing approach to uncover the miRNOME in the human heart. PLoS One 8(2):e57800
Nandi SS, Zheng H, Sharma NM, Shahshahan HR, Patel KP, Mishra PK (2016) Lack of miR-133a decreases contractility of diabetic hearts: a role for novel cross talk between tyrosine aminotransferase and tyrosine hydroxylase. Diabetes 65(10):3075–3090
Shahshahan HR, Kambis TN, Kar S, Yadav SK, Mishra PK (2021) Generating Ins2(+/−)/miR-133aTg mice to model miRNA-driven cardioprotection of human diabetic heart. Methods Mol Biol 2224:113–121
Kambis TN, Shahshahan HR, Kar S, Yadav SK, Mishra PK (2019) Transgenic expression of miR-133a in the diabetic Akita heart prevents cardiac remodeling and cardiomyopathy. Front Cardiovasc Med 6:45
Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ (2011) Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol 29(4):341–345
De Leo V, Milano F, Agostiano A, Catucci L (2021) Recent advancements in polymer/liposome assembly for drug delivery: from surface modifications to hybrid vesicles. Polymers (Basel) 13(7):1027
Chenthamara D, Subramaniam S, Ramakrishnan SG, Krishnaswamy S, Essa MM, Lin FH, Qoronfleh MW (2019) Therapeutic efficacy of nanoparticles and routes of administration. J Biomed Mater Res 23:20
Sung YK, Kim SW (2019) Recent advances in the development of gene delivery systems. J Biomed Mater Res 23:8. https://doi.org/10.1186/s40824-019-0156-z
Nayerossadat N, Maedeh T, Ali PA (2012) Viral and nonviral delivery systems for gene delivery. Adv Biomed Res 1:27
Thomas CE, Ehrhardt A, Kay MA (2003) Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet 4(5):346–358
Naso MF, Tomkowicz B, Perry WL 3rd, Strohl WR (2017) Adeno-associated virus (AAV) as a vector for gene therapy. BioDrugs 31(4):317–334
Greenberg B, Yaroshinsky A, Zsebo KM, Butler J, Felker GM, Voors AA, Rudy JJ, Wagner K, Hajjar RJ (2014) Design of a phase 2b trial of intracoronary administration of AAV1/SERCA2a in patients with advanced heart failure: the CUPID 2 trial (calcium up-regulation by percutaneous administration of gene therapy in cardiac disease phase 2b). JACC Heart Fail 2(1):84–92
Greenberg B, Butler J, Felker GM, Ponikowski P, Voors AA, Desai AS, Barnard D, Bouchard A, Jaski B, Lyon AR, Pogoda JM, Rudy JJ, Zsebo KM (2016) Calcium upregulation by percutaneous administration of gene therapy in patients with cardiac disease (CUPID 2): a randomised, multinational, double-blind, placebo-controlled, phase 2b trial. Lancet 387(10024):1178–1186
Di Pasquale E, Latronico MV, Jotti GS, Condorelli G (2012) Lentiviral vectors and cardiovascular diseases: a genetic tool for manipulating cardiomyocyte differentiation and function. Gene Ther 19(6):642–648
Niwano K, Arai M, Koitabashi N, Watanabe A, Ikeda Y, Miyoshi H, Kurabayashi M (2008) Lentiviral vector-mediated SERCA2 gene transfer protects against heart failure and left ventricular remodeling after myocardial infarction in rats. Mol Ther 16(6):1026–1032
Ly H, Kawase Y, Yoneyama R, Hajjar RJ (2007) Gene therapy in the treatment of heart failure. Physiology (Bethesda) 22:81–96
Nandi SS, Shahshahan HR, Shang Q, Kutty S, Boska M, Mishra PK (2018) MiR-133a mimic alleviates T1DM-induced systolic dysfunction in Akita: an MRI-based study. Front Physiol 9:1275
Rincon MY, VandenDriessche T, Chuah MK (2015) Gene therapy for cardiovascular disease: advances in vector development, targeting, and delivery for clinical translation. Cardiovasc Res 108(1):4–20
Acknowledgment
This work was supported in part by the National Institutes of Health (NIH) grant F31HL156402 to TNK and pilot projects of Nebraska Center for the Prevention of Obesity Diseases from NIH P20GM104320 and of UNMC Center for Heart and Vascular Research from the National Institute of General Medical Sciences 1U54GM115458 to PKM. The content is solely the responsibility of the author and does not necessarily represent the official view of the NIH.
Authors’ Contribution: TNK prepared Fig. 2 and contributed in editing and revising manuscript. PKM drafted the manuscript and prepared Fig. 1.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
No conflict of interest to disclose.
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Kambis, T.N., Mishra, P.K. (2023). Genome Editing and Diabetic Cardiomyopathy. In: **ao, J. (eds) Genome Editing in Cardiovascular and Metabolic Diseases. Advances in Experimental Medicine and Biology, vol 1396. Springer, Singapore. https://doi.org/10.1007/978-981-19-5642-3_7
Download citation
DOI: https://doi.org/10.1007/978-981-19-5642-3_7
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-5641-6
Online ISBN: 978-981-19-5642-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)