Abstract
Rectal prolapse (RP) is a disabling condition and can range from internal rectal prolapse (IRP) or rectal intussusception to full-thickness external rectal prolapse (ERP). RP occurs in 0.5% of the general population, with a higher incidence in females and the elderly [1].Intellectual disability and psychiatric conditions are a risk factor for RP in younger patients. Patients with an IRP usually experience functional symptoms of obstructed defecation (OD) or fecal incontinence (FI), while patients with ERP suffer from pain, rectal bleeding, and FI [2].Two recent guidelines have been published on the management of rectal prolapse, the 2017 American guidelines [1] and the 2017 Dutch guidelines [2].The recommendations in this chapter are summarized from these sets of guidelines as well as additional up-to-date evidence.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Introduction
Rectal prolapse (RP) is a disabling condition and can range from internal rectal prolapse (IRP) or rectal intussusception to full-thickness external rectal prolapse (ERP). RP occurs in 0.5% of the general population, with a higher incidence in females and the elderly [1].Intellectual disability and psychiatric conditions are a risk factor for RP in younger patients. Patients with an IRP usually experience functional symptoms of obstructed defecation (OD) or fecal incontinence (FI), while patients with ERP suffer from pain, rectal bleeding, and FI [2].Two recent guidelines have been published on the management of rectal prolapse, the 2017 American guidelines [1] and the 2017 Dutch guidelines [2].The recommendations in this chapter are summarized from these sets of guidelines as well as additional up-to-date evidence.
The evaluation of a patient with suspected RP should include a thorough history and physical examination. Bowel symptom severity should be graded using validated questionnaires for constipation or fecal incontinence. Up to a third of patients with RP have concomitant symptoms of anterior compartment prolapse, including urinary incontinence and vaginal vault prolapse. Decreased anal sphincter tone is often present, and proctoscopy may show an anterior solitary rectal ulcer in 10–15% of cases. Straining in the squatting position may be required to induce ERP.
If the diagnosis of IRP is uncertain, fluoroscopic or MRI defecography should be performed. A transit marker study can be considered to assess symptoms of slow transit constipation, which is a relative contraindication to surgery. Anorectal function tests including manometry and anorectal physiology studies may be useful if IRP is suspected based on bowel symptoms, and results may alter management. In patients with OD, pelvic floor muscle dyssynergia is a contraindication to rectopexy. ERP is an absolute indication for surgery; therefore, imaging and anorectal function tests generally do not add any further value to management. Patients with various functional symptoms arising from multicompartment prolapse should be discussed in a multidisciplinary setting to achieve optimal decision-making. For selected patients, psychiatric evaluation may be necessary to exclude a psychosomatic origin of symptoms. Endoscopic colonic evaluation should be performed to rule out malignancy. Patients with functional symptoms from IRP must be considered for conservative therapy, including lifestyle modification, pharmacological treatment, pelvic floor physiotherapy, and retrograde colonic irrigation, if available.
Choice of Surgery
Although associated symptoms can be alleviated with conservative management, RP cannot be corrected without surgery. Surgical intervention should be tailored to the patient’s overall health status, concomitant pelvic organ prolapse, and history of previous procedures. A host of different techniques have been described in the literature, with two main approaches, perineal versus transabdominal. The choice between the two is usually determined by the surgeon’s preference and experience as well as the patient’s comorbidities and bowel function. The most performed perineal methods are the perineal rectosigmoidectomy (Altemeier procedure) and perineal mucosal sleeve resection with muscular plication (Delorme procedure). The most common transabdominal techniques are the anterior rectopexy with or without sigmoid resection, and the ventral mesh rectopexy.
Previous evidence reported that transabdominal approaches resulted in lower recurrence rates and better functional outcomes compared to a perineal approach [1]. However, a 2015 Cochrane review of 15 randomized trials involving 1007 patients was unable to demonstrate a significant difference in the recurrence rate between an abdominal or perineal technique [3]. A 2015 randomized trial comparing laparoscopic ventral mesh rectopexy versus the Delorme procedure similarly did not show a statistically significant difference in the incidence of recurrence or complication rates [4].
Open rectopexy is associated with higher postoperative morbidity compared to laparoscopic or perineal surgery. The well-documented advantages of minimally invasive surgery in the early postoperative period also make laparoscopy preferred over open rectopexy. Laparoscopic ventral mesh rectopexy (LVMR) was described by D’Hoore et al. in 2004; [5] this technique was autonomic nerve-sparing, addressed the anterior lead point of an IRP, and corrected a concomitant rectocele, resulting in significant improvement in postoperative constipation and no incidence of de novo constipation.
A recent 2018 meta-analysis [4] of 17 studies (13 retrospective studies, three randomized trials, and one prospective cohort study) with 1242 patients undergoing LVMR for ERP showed a mean complication rate of 12.4%, with a mean ERP recurrence rate of 2.8% over a median follow-up duration of 23 months, and mean rates of improvement in fecal incontinence and constipation of 79.3% and 71%, respectively. Median operating time was about 120 min and conversion to open surgery was necessary in 1.8% of patients. Acceptable long-term outcomes have also been published; the 10 year recurrence rate following LVMR was 8.2% with a 4.6% mesh-related complication rate (1.3% vaginal mesh erosion) in 919 patients [6]. 76% of patients reported subjective functional symptom relief at a median follow-up time of 44 months from surgery [7].
In view of these findings, the LVMR has become the most popular laparoscopic technique for RP, particularly in Europe. The 2017 Dutch guidelines recommend the LVMR as the first-choice procedure for ERP as well as IRP with an indication for surgery [2]. Although resection rectopexy is thought by some to improve symptoms of constipation in patients with a redundant sigmoid colon, there is no evidence favoring it over LVMR and the risk of an anastomotic leak must be considered. The robotic-assisted LVMR is similar to LVMR in terms of functional outcome, complication, and recurrence rates, although it requires a longer operative time and increased costs [8, 9]. Further evidence is required to determine if the potential technical benefits of robotic surgery translate to better clinical outcomes.
OT Setup
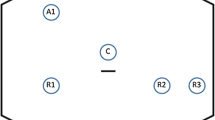
Bowel preparation using 2 L polyethylene glycol is used. Below-knee compression stockings are applied and a urinary catheter is inserted. The patient is placed in the modified Lloyd-Davis position with the lower limbs in foot stirrups. Both arms are tucked in to facilitate positioning of the surgeon, camera operator, and assistant. A small sandbag (or folded drapes) is placed below the sacrum to elevate the pelvis approximately 4–5 cm anteriorly to enable better visualization of the deep pelvic structures during surgery. A single dose of prophylactic intravenous antibiotics is given at anesthetic induction. A schematic of the operating setup and port positioning is shown in Fig. 1. A 12 mm camera trocar is placed at the umbilicus. A 12 mm trocar is placed at the right iliac fossa and two 5 mm trocars are placed at the right and left flanks.
Surgical Technique
Essential steps and technique
-
1.
Dissection
-
2.
Mesh fixation
-
3.
Vaginal fornix fixation
-
4.
Neo-Douglas formation
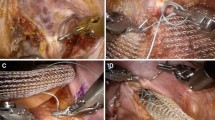
We use the technique as described by D’Hoore for LVMR in 2006 [10]. With the patient in a steep Trendelenburg position, the uterus is temporarily hitched to the anterior abdominal wall using a Prolene 2–0 straight needle passed through the uterus fundus or broad ligaments (Fig. 2). With the surgical assistant providing traction on the sigmoid out of the pelvis and to the left, the peritoneum is incised from the sacral promontory to the pouch of Douglas (Fig. 3). The rectum is not mobilized laterally or posteriorly, and the right hypogastric nerve is preserved, decreasing injury to the parasympathetic and sympathetic rectal innervation. The rectovaginal septum is carefully opened down to the pelvic floor, avoiding injury to the rectum (Fig. 4).
Choice of mesh is an important consideration. In D’Hoore’s original description, a 3 × 17 cm polypropylene mesh was used [10]. A 2017 systematic review of eight studies from 2004 to 2015 compared 3517 patients using synthetic mesh and 439 patients using biological mesh for LVMR, with the rates of mesh-related erosion at 1.9% and 0.2%, respectively [11]. The largest series of biological mesh used with the longest follow-up was published in 2017 [12]. Of 224 patients who underwent LVMR using Permacol™ biological mesh, mesh-related morbidity was 0.5%, with a 11.4% recurrence rate. There was significantly improved constipation, fecal incontinence, quality of life outcomes, and associated improvement in urogynecological symptoms.
We use a 10 × 10 cm, 1 mm thick Permacol mesh, cut and stitched together using Prolene 2–0 sutures to fashion a [3, 13] × (15–20) cm strip of mesh. The mesh must be long enough to allow the distal end to reach the anterior rectal pelvic floor and the proximal end to be secured to the sacral promontory. If available, the 4 × 18 cm 1 mm thick Permacol mesh will be better suited for this purpose. The mesh is sutured to the distal rectum using Prolene 2–0, with four sutures on each ventrolateral edge of the rectum (Fig. 5). The sutures are applied with the aid of a knot-pusher from distal to proximal at approximately 1 cm intervals, beginning at the level of the pelvic floor. Although the RP should be reduced at the time of mesh fixation, no traction should be exerted on the rectum, which remains along the curve of the sacrococcygeal hollow [10]. The mesh is fixed onto the sacral promontory using a laparoscopic tacker (Fig. 6). Next, the posterior vaginal fornix is sutured onto the distal aspect of the mesh. This closes the rectovaginal septum and corrects a rectocele (Fig. 7). Excess proximal mesh is trimmed following fixation (Fig. 8).
The peritoneum is closed over the mesh using a continuous PDS 2–0 suture, commencing from the left lateral edge of the peritoneal incision to the sacral promontory (Fig. 9). The pouch of Douglas is recreated by approximating the peritoneum to the rectal serosa above the rectovaginal colpopexy. In this manner, the entire mesh is covered, preventing future adhesion to the small bowel (Fig. 9). The uterine hitch is removed. No abdominal drain is placed. Finally, the abdominal wounds are closed, concluding the surgery.
Complications and Management
A 2015 study looked at data from 2203 patients from five centers undergoing LVMR from 1999 to 2013 [14]. Synthetic mesh was used in 80% of patients versus biological mesh in 20%. Non-mesh morbidity occurred in 11% (including pain, port site complications, urinary retention, or infection). The overall rate of mesh erosion was 2.0% (2.4% synthetic mesh and 0.7% biologic mesh), including 20 vaginal, 17 rectal, 7 rectovaginal fistula, and 1 perineal, at a median time to erosion of 23 months. Of patients who suffered mesh erosion, 50% required treatment for minor erosion morbidity including local excision of stitch or exposed mesh. 40% underwent intervention for major erosion morbidity including operative mesh removal, colostomy creation, and anterior resection of rectum.
Postoperative Care
No further antibiotics are given beyond the induction dose. The urinary catheter can be removed on postoperative day 1 or 2, and the patient is discharged following bowel motion. The patient is advised to avoid excessive straining, and a course of stool bulking agents may be required.
References
Bordeianou L, Paquette I, Johnson E, Holubar SD, Gaertner W, Feingold DL, Steele SR. Clinical practice guidelines for the treatment of rectal prolapse. Dis Colon Rectum. 2017;60(11):1121.
van der Schans EM, Paulides TJC, Wijffels NA, Consten ECJ. Management of patients with rectal prolapse: the 2017 Dutch guidelines. Tech Coloproctol. 2018;22(8):589–96.
Tou S, Brown SR, Nelson RL. Surgery for complete (full-thickness) rectal prolapse in adults. Cochrane Database Syst Rev. 2015;2015(11):CD001758.
Emile SH, Elfeki H, Shalaby M, Sakr A, Sileri P, Wexner SD. Outcome of laparoscopic ventral mesh rectopexy for full-thickness external rectal prolapse: a systematic review, meta-analysis, and meta-regression analysis of the predictors for recurrence. Surg Endosc. 2019;33(8):2444–55.
D'Hoore A, Cadoni R, Penninckx F. Long-term outcome of laparoscopic ventral rectopexy for total rectal prolapse. Br J Surg. 2004;91(11):1500–5.
Consten EC, van Iersel JJ, Verheijen PM, Broeders IA, Wolthuis AM, D’Hoore A. Long-term outcome after laparoscopic ventral mesh Rectopexy: an observational study of 919 consecutive patients. Ann Surg. 2015;262(5):742–7; discussion 747-8
Mäkelä-Kaikkonen J, Rautio T, Kairaluoma M, Carpelan-Holmström M, Kössi J, Rautio A, Ohtonen P, Mäkelä J. Does ventral Rectopexy improve pelvic floor function in the long term? Dis Colon Rectum. 2018;61(2):230–8.
Ramage L, Georgiou P, Tekkis P, Tan E. Is robotic ventral mesh rectopexy better than laparoscopy in the treatment of rectal prolapse and obstructed defecation? A meta-analysis. Tech Coloproctol. 2015;19(7):381–9.
Albayati S, Chen P, Morgan MJ, Toh JWT. Robotic vs. laparoscopic ventral mesh rectopexy for external rectal prolapse and rectal intussusception: a systematic review. Tech Coloproctol. 2019;23(6):529–35.
D'Hoore A, Penninckx F. Laparoscopic ventral recto(colpo)pexy for rectal prolapse: surgical technique and outcome for 109 patients. Surg Endosc. 2006;20(12):1919–23.
Balla A, Quaresima S, Smolarek S, Shalaby M, Missori G, Sileri P. Synthetic versus biological mesh-related erosion after laparoscopic ventral mesh Rectopexy: a systematic review. Ann Coloproctol. 2017;33(2):46–51.
McLean R, Kipling M, Musgrave E, Mercer-Jones M. Short- and long-term clinical and patient-reported outcomes following laparoscopic ventral mesh rectopexy using biological mesh for pelvic organ prolapse: a prospective cohort study of 224 consecutive patients. Color Dis. 2018;20(5):424–36.
Emile SH, Elbanna H, Youssef M, Thabet W, Omar W, Elshobaky A, Abd El-Hamed TM, Farid M. Laparoscopic ventral mesh rectopexy vs Delorme's operation in management of complete rectal prolapse: a prospective randomized study. Color Dis. 2017;19(1):50–7.
Evans C, Stevenson AR, Sileri P, Mercer-Jones MA, Dixon AR, Cunningham C, Jones OM, Lindsey I. A multicenter collaboration to assess the safety of laparoscopic ventral Rectopexy. Dis Colon Rectum. 2015;58(8):799–807.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this chapter
Cite this chapter
Seow-En, I., Kwong-Wei, E., Chen, WL. (2023). Laparoscopic Ventral Mesh Rectopexy. In: Lomanto, D., Chen, W.TL., Fuentes, M.B. (eds) Mastering Endo-Laparoscopic and Thoracoscopic Surgery. Springer, Singapore. https://doi.org/10.1007/978-981-19-3755-2_74
Download citation
DOI: https://doi.org/10.1007/978-981-19-3755-2_74
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-3754-5
Online ISBN: 978-981-19-3755-2
eBook Packages: MedicineMedicine (R0)