Abstract
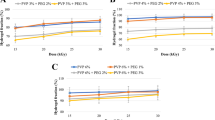
Donepezil hydrochloride, an anticholinesterase drug, is orally administered to patients suffering from Alzheimer’s disease. Alzheimer’s is a neurodegenerative disorder characterized by the degradation of neurotransmitters, neuronal apoptosis, and loss of synapses. The treatment of such neural diseases is hampered by the presence of a tightly controlled blood–brain barrier (BBB), which prevents the influx of drugs. The intranasal route of drug delivery has been lately seen as a promising approach to deliver drugs directly to the brain, effectively bypassing the BBB, as it offers high absorption and increased bioavailability. In this study, we have fabricated hybrid polymeric lipid nanoparticles with two natural polymers—chitosan and gelatin. The average particle sizes of chitosan lecithin and gelatin lecithin nanoparticles were 237.43 and 278.86 nm. The percentage drug loading for chitosan lecithin and gelatin lecithin nanoparticles was 10.24 ± 0.4 and 8.77 ± 0.748%. The chitosan lecithin nanoparticles showed a burst release of up to 99.99% drug for 5 days, while the gelatin lecithin nanoparticles exhibited a sustained release of 33.31% drug for 30 days under acidic conditions. The cell viability studies showed that both nanoparticles were safe toward mouse fibroblast cells (L929). Finally, both nanoparticles were found to be mucoadhesive in nature. Out of the two nanoparticulate systems developed, the gelatin lecithin nanoparticles demonstrates a strong potential as a carrier for donepezil delivery.
Meghana Bhandari and Nahida Rasool-both authors contributed equally to the work
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Nisbet RM, Götz J (2018) Amyloid-β and Tau in Alzheimer’s disease: novel pathomechanisms and non-pharmacological treatment strategies. J Alzheimers Dis 64(s1):S517–S527. https://doi.org/10.3233/JAD-179907
(2020) Alzheimer’s disease facts and figures. Alzheimer’s Dement 16(3):391–460
NIH National Institute on Aging. https://www.nia.nih.gov
Jelic V, Darreh-Shori T (2010) Donepezil: a review of pharmacological characteristics and role in the management of Alzheimer disease. Clin Med Insights: Ther 2:771–788. https://doi.org/10.4137/CMT.S5410
Devnarain N, Ramharack P, Soliman ME (2017) Brain grants permission of access to Zika virus but denies entry to drugs: a molecular modeling perspective to infiltrate the boundary. RSC Adv 7(75):47416–47424. https://doi.org/10.1039/C7RA05918C
Liew KB, Tan YTF, Peh KK (2012) Characterization of oral disintegrating film containing donepezil for Alzheimer disease. AAPS PharmSciTech 13(1):134–142. https://doi.org/10.1208/s12249-011-9729-4
Fonseca LC, Lopes JA, Vieira J et al (2021) Intranasal drug delivery for treatment of Alzheimer’s disease. Drug Deliv Transl Res 11(2):411–425. https://doi.org/10.1007/s13346-021-00940-7
Pires PC, Santos AO (2018) Nanosystems in nose-to-brain drug delivery: a review of non-clinical brain targeting studies. J Control Release 270:89–100. https://doi.org/10.1016/j.jconrel.2017.11.047
Yousfan A, Rubio N, Natouf AH et al (2020) Preparation and characterization of PHT-loaded chitosan lecithin nanoparticles for intranasal drug delivery to the brain. RSC Adv 10(48):28992–29009. https://doi.org/10.1039/D0RA04890A
Elzoghby AO (2013) Gelatin-based nanoparticles as drug and gene delivery systems: reviewing three decades of research. J Control Release 172(3):1075–1091. https://doi.org/10.1016/j.jconrel.2013.09.019
Kommareddy S, Shenoy DB, Amiji MM (2007) Gelatin nanoparticles and their biofunctionalization. Nanotechnologies Life Sci. https://doi.org/10.1002/9783527610419.ntls0011
Abbasiliasi S, Shun TJ, Tengku Ibrahim TA et al (2019) Use of sodium alginate in the preparation of gelatin-based hard capsule shells and their evaluation: in vitro. RSC Adv 9(28):16147–16157. https://doi.org/10.1039/C9RA01791G
Hafner A, Lovrić J, Pepić I, Filipović-Grčić J (2011) Lecithin/chitosan nanoparticles for transdermal delivery of melatonin. J Microencapsul 28(8):807–815. https://doi.org/10.3109/02652048.2011.622053
Chhonker YS, Prasad YD, Chandasana H et al (2015) Amphotericin-B entrapped lecithin/chitosan nanoparticles for prolonged ocular application. Int J Biol Macromol 72:1451–1458. https://doi.org/10.1016/j.ijbiomac.2014.10.014
Pineda-Álvarez RA, Bernad-Bernad MJ, Rodríguez-Cruz IM et al (2020) Development and characterization of starch/gelatin microneedle arrays loaded with lecithin-gelatin nanoparticles of losartan for transdermal delivery. J Pharm Innov. https://doi.org/10.1007/s12247-020-09494-6
Jain S, Valvi PU, Swarnakar NK, Thanki K (2012) Gelatin coated hybrid lipid nanoparticles for oral delivery of Amphotericin B. Mol Pharm 9(9):2542–2553. https://doi.org/10.1021/mp300320d
Zhang L, Chan JM, Gu FX et al (2008) Self-assembled lipid-polymer hybrid nanoparticles: a robust drug delivery platform. ACS Nano 2(8):1696–1702. https://doi.org/10.1021/nn800275r
Gajendiran M, Jainuddin Yousuf SM, Elangovan V, Balasubramanian S (2014) Gold nanoparticle conjugated PLGA-PEG-SA-PEG-PLGA multiblock copolymer nanoparticles: synthesis, characterization, in vivo release of rifampicin. J Mater Chem B, Mater Biol Med 2(4):418–427. https://doi.org/10.1039/C3TB21113D
Perez-Ruiz AG, Ganem A, Olivares-Corichi IM, García-Sánchez JR (2018) Lecithin-chitosan-TPGS nanoparticles as nanocarriers of (-)-epicatechin enhanced its anticancer activity in breast cancer cells. RSC Adv 8(61):34773–34782. https://doi.org/10.1021/nn800275r
Foo JB, Ng LS, Lim JH, Tan PX, Lor YZ, Loo JSE, Low ML, Chan LC, Beh CY, Leong SW, Saiful Yazan L, Tor YS, How CW (2019) Induction of cell cycle arrest and apoptosis by copper complex Cu(SBCM)2 towards oestrogen-receptor positive MCF-7 breast cancer cells. RSC Adv 9(32):18359–18370. https://doi.org/10.1039/C9RA03130H
Lee JS, Suh JW, Kim ES, Lee HG (2017) Preparation and characterization of mucoadhesive nanoparticles for enhancing cellular uptake of coenzyme Q10. J Agric Food Chem 65(40):8930–8937. https://doi.org/10.1021/acs.jafc.7b03300
Gonçalves VSS, Poejo J, Matias AA, Rodríguez-Rojo S, Cocero MJ, Duarte CMM (2016) Using different natural origin carriers for development of epigallocatechin gallate (EGCG) solid formulations with improved antioxidant activity by PGSS-drying. RSC Adv 6(72):67599–67609. https://doi.org/10.1039/C6RA13499H
Acknowledgements
Financial assistance from the DBT India to Y.S. (BT/PR40669/MED/32/761/2020) is gratefully acknowledged. We also thank the Departments of Biomedical Engineering and Chemistry for providing the access to their research facilities. M.B. and N.R. received their fellowships from IIT Ropar.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Bhandari, M., Rasool, N., Singh, Y. (2022). Polymeric Lipid Nanoparticles for Donepezil Delivery. In: Gupta, B., Jawaid, M., Kaith, B.S., Rattan, S., Kalia, S. (eds) Polymeric Biomaterials and Bioengineering. Lecture Notes in Bioengineering. Springer, Singapore. https://doi.org/10.1007/978-981-19-1084-5_5
Download citation
DOI: https://doi.org/10.1007/978-981-19-1084-5_5
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-1083-8
Online ISBN: 978-981-19-1084-5
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)