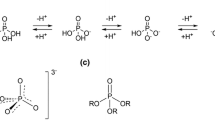
Abstract
Internucleotide phosphate diester bonds in unmodified oligonucleotides are rapidly degraded by nucleolytic enzymes in cells or body fluids. This property excludes natural DNA and RNA molecules from potential medical applications and from many structural and mechanistic studies. DNA nucleotides and oligonucleotides in which one of the nonbridging phosphate oxygen atoms is replaced by a sulfur atom (PS-DNA) were among the first DNA analogs to be designed and synthesized. PS-DNA exhibits significantly higher nuclease resistance and also offers important opportunities for detailed studies of interactions with other biomolecules at the molecular level. However, the substitution creates a stereogenic center at the phosphorus atom, so that even short oligomers synthesized by a non-stereocontrolled method exist as mixtures of hundreds or even thousands of P-diastereomers, which usually cannot be separated chromatographically. Stereocontrolled synthesis methods have been developed to overcome this problem. P-stereodefined probes, including isotopomerically labeled species, have been used to elucidate the mode of action of numerous enzymes (nucleases, transferases, and kinases), ribozymes, and DNA-zymes, as well as to study the thermodynamic stability of nucleic acid complexes (duplexes, triplexes, and i-motif) and the mechanism of B-Z-type conformational changes. They are also useful tools for tuning the properties of siRNA duplexes.
For many years, phosphorothioate modification was considered purely artificial, having been designed and implemented by chemists. However, in 2007, a phosphorothioate modification of DNA in bacteria was discovered and its functioning was intensively studied. In 2020, a report was published on the presence of a phosphorothioate modification in RNA isolated from prokaryotes and eukaryotes, but this claim has been criticized and seems premature, to say the least.
This chapter covers two main areas related to PS-oligonucleotides: first, the synthetic routes to P-stereodefined oligonucleotides and selected examples of their application in structural, biochemical, and biological experiments; and second, the biosynthesis of oligonucleotides with phosphorothioate modification and their physiological functions.
Similar content being viewed by others
References
An X, **ong W, Yang Y, Li F, Zhou X, Wang Z, Deng Z, Liang J (2012) A novel target of IscS in Escherichia coli: participating in DNA phosphorothioation. PLoS One 7:e51265. https://doi.org/10.1371/journal.pone.0051265
Auffinger P, Westhof E (2001) RNA solvation: a molecular dynamics simulation perspective. Biopolymers 56:266–274. https://doi.org/10.1002/1097-0282(2000)56:4%3C266::AID-BIP10027%3E3.0.CO;2-3
Bailey JK, Shen W, Liang XH, Crooke ST (2017) Nucleic acid binding proteins affect the subcellular distribution of phosphorothioate antisense oligonucleotides. Nucleic Acids Res 45:10649–10671. https://doi.org/10.1093/nar/gkx709
Benimetskaya L, Tonkinson JL, Koziołkiewicz M, Karwowski B, Guga P, Zelser R, Stec WJ, Stein CA (1995) Binding of phosphorothioate oligonucleotides to basic fibroblast growth factor, recombinant soluble CD4, Laminin and fibronectin is P-chirality independent. Nucleic Acids Res 23:4239–4245
Berk C, Civenni G, Wang Y, Steuer C, Catapano CV, Hall J (2021) Pharmacodynamic and pharmacokinetic properties of full phosphorothioate small interfering RNAs for gene silencing in vivo. Nucleic Acid Ther 31:237–244. https://doi.org/10.1089/nat.2020.0852
Biscans A, Caiazzi J, Davis S, McHugh N, Sousa J, Khvorova A (2020) The chemical structure and phosphorothioate content of hydrophobically modified siRNAs impact extrahepatic distribution and efficacy. Nucleic Acids Res 48:7665–7680. https://doi.org/10.1093/nar/gkaa595
Boczkowska M, Guga P, Karwowski B, Maciaszek A (2000) Effect of P-chirality of internucleotide bonds on B-Z conversion of stereodefined selfcomplementary phosphorothioate oligonucleotides of [PS]-d(CG)4 and [PS]-d(GC)4 series. Biochemistry 39:11057–11064. https://doi.org/10.1021/bi000638n
Boczkowska M, Guga P, Stec WJ (2002) Stereodefined phosphorothioate analogues of DNA: relative thermodynamic stability of model PS-DNA/DNA and PS-DNA/RNA complexes. Biochemistry 41:12483–12487. https://doi.org/10.1021/bi026225z
Braasch DA, Paroo Z, Constantinescu A, Ren G, Oz OK, Mason RP, Corey DR (2004) Biodistribution of phosphodiester and phosphorothioate siRNA. Bioorg Med Chem Lett 14:1139–1143. https://doi.org/10.1016/j.bmcl.2003.12.074
Breaker RR (2000) Making catalytic DNAs. Science 290:2095–2096. https://doi.org/10.1126/science.290.5499.2095
Cao B, Cheng Q, Gu C, Yao F, DeMott MS, Zheng X, Deng Z, Dedon PC, You D (2014) Pathological phenotypes and in vivo DNA cleavage by unrestrained activity of a phosphorothioate-based restriction system in salmonella. Mol Microbiol 93:776–785. https://doi.org/10.1111/mmi.12692
Crooke ST (ed) (1998) Handbook of experimental pharmacology: antisense research and applications, vol 131. Springer, Berlin/Heidelberg
Crooke ST, Wang S, Vickers TA, Shen W, Liang X (2017) Cellular uptake and trafficking of antisense oligonucleotides. Nat Biotechnol 35:230–237. https://doi.org/10.1038/nbt.3779
Dertinger D, Behlen LS, Uhlenbeck OC (2000) Using phosphorothioate-substituted RNA to investigate the thermodynamic role of phosphates in a sequence specific RNA-protein complex. Biochemistry 39:55–63. https://doi.org/10.1021/bi991769v
Detzer A, Sczakiel G (2009) Phosphorothioate-stimulated uptake of siRNA by mammalian cells: a novel route for delivery. Curr Top Med Chem 9:1109–1116. https://doi.org/10.2174/15680260978963088
Detzer A, Overhoff M, Mescalchin A, Rompf M, Sczakiel G (2008) Phosphorothioate-stimulated cellular uptake of siRNA: a cell culture model for mechanistic studies. Curr Pharm Des 14:3666–3673. https://doi.org/10.2174/138161208786898770
Eckstein F (2000) Phosphorothioate oligodeoxynucleotides: what is their origin and what is unique about them? Antisense Nucleic Acid Drug Dev 10:117–121. https://doi.org/10.1089/oli.1.2000.10.117
Egli M, Tereshko V, Teplova M, Minasov G, Joachimiak A, Sanishvili R, Weeks CM, Miller R, Maier MA, An H, Dan Cook P, Manoharan M (1998) X-ray crystallographic analysis of the hydration of A- and B-form DNA at atomic resolution. Biopolymers 48:234–252. https://doi.org/10.1002/(SICI)1097-0282(1998)48:4%3C234::AID-BIP4%3E3.0.CO;2-H
Fearon KL, Hirschbein BL, Chiu C-Y, Quijano MR, Zon G (1997) Phosphorothioate oligodeoxynucleotides: large scale synthesis and analysis, impurity characterization, and the effects of phosphorus stereochemistry. Ciba Foundation Symp 209:19–31. https://doi.org/10.1002/9780470515396.ch3
Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC (1998) Potent and specific genetic interference by doublestranded RNA in Caenorhabditis elegans. Nature 391:806–811. https://doi.org/10.1038/35888
Flynn LL, Li R, Pitout IL, Aung-Htut MT, Larcher LM, Cooper JAL, Greer KL, Hubbard A, Griffiths L, Bond CS, Wilton SD, Fox AH, Fletcher S (2022) Single stranded fully modified-phosphorothioate oligonucleotides can induce structured nuclear inclusions, alter nuclear protein localization and disturb the transcriptome in vitro. Front Genet 6:791416. https://doi.org/10.3389/fgene.2022.791416
Frey PA, Sammons RD (1985) Bond order and charge localization in nucleoside phosphorothioates. Science 228:541–545. https://doi.org/10.1126/science.2984773
Guga P, Koziołkiewicz M (2011) Phosphorothioate nucleotides and oligonucleotides – recent progress in synthesis and application. Chem Biodivers 8:1642–1681. https://doi.org/10.1002/cbdv.201100130
Guga P, Stec WJ (2003) Synthesis of phosphorothioate oligonucleotides with Stereodefined Phosphorothioate linkages. In: Beaucage SL, Bergstrom DE, Glick GD, Jones RA (eds) Current protocols in nucleic acid chemistry. Wiley, Hoboken, pp 4.17.1–4.17.28. https://doi.org/10.1002/0471142700.nc0417s14
Guga P, Boczkowska M, Janicka M, Maciaszek A, Kuberski S, Stec WJ (2007a) Unusual thermal stability of RNA/[All-RP-PS]-DNA/RNA triplexes containing a Homopurine DNA Strand. Biophys J 92:2507–2515. https://doi.org/10.1529/biophysj.106.099283
Guga P, Janicka M, Maciaszek A, Rębowska B, Nowak G (2007b) Hoogsteen paired Homopurine [RP-PS]-DNA and homopyrimidine RNA strands form a thermally stable parallel duplex. Biophys J 93:3567–3574. https://doi.org/10.1529/biophysj.107.108183
Horton TE, Maderia M, DoRose VJ (2000) Impact of phosphorothioate substitutions on the thermodynamic stability of an RNA GAAA Tetraloop: an unexpected stabilization. Biochemistry 39:8201–8207. https://doi.org/10.1021/bi000141d
Huang Q, Li R, Yi T, Cong F, Wang D, Deng Z, Zhao YL (2021) Phosphorothioate-DNA bacterial diet reduces the ROS levels in C. elegans while improving locomotion and longevity. Commun Biol 4:1335. https://doi.org/10.1038/s42003-021-02863-y
Iwamoto N, Butler D, Svrzikapa N, Mohapatra S, Zlatev I, Sah DWY, Meena SSM, Lu G, Apponi LH, Frank-Kamenetsky M, **gxin Zhang J, Vargeese C, Verdine GL (2017) Control of phosphorothioate stereochemistry substantially increases the efficacy of antisense oligonucleotides. Nat Biotechnol 35:845–851. https://doi.org/10.1038/nbt.3948
Jahns H, Roos M, Imig J, Baumann F, Wang Y, Gilmour R, Hall J (2015) Stereochemical bias introduced during RNA synthesis modulates the activity of phosphorothioate siRNAs. Nat Commun 6:6317. https://doi.org/10.1038/ncomms7317
Jahns H, Taneja N, Willoughby JLS, Akabane-Nakata M, Brown CR, Nguyen T, Bisbe A, Matsuda S, Hettinger MRM, Rajeev KG, Maier MA, Zlatev I, Charisse K, Egli M, Manoharan M (2022) Chirality matters: stereo-defined phosphorothioate linkages at the termini of small interfering RNAs improve pharmacology in vivo. Nucleic Acids Res 50:1221–1240. https://doi.org/10.1093/nar/gkab544
Jaroszewski JW, Syi J-L, Maizel J, Cohen JS (1992) Towards rational design of antisense DNA: molecular modelling of phosphorothioate DNA analogues. Anticancer Drug Des 7:253–262
Jastrzębska K, Maciaszek A, Dolot R, Bujacz G, Guga P (2015) Thermal stability and conformation of antiparallel duplexes formed by P-stereodefined phosphorothioate DNA/LNA chimeric oligomers with DNA and RNA matrices. Org Biomol Chem 13:10032–10040. https://doi.org/10.1039/c5ob01474c
Jastrzębska K, Mikołajczyk B, Guga P (2020) LNA units present in [RP-PS]-(DNA#LNA) chimeras enhance the thermal stability of parallel duplexes and triplexes formed with (2′-OMe)-RNA strands. RSC Adv 10:22370–22376. https://doi.org/10.1039/d0ra03934a
Jastrzębska K, Maciaszek A, Dolot R, Tomaszewska-Antczak A, Mikołajczyk B, Guga P (2022) Synthesis and hybridizing properties of P-stereodefined chimeric [PS]-{DNA:RNA} and [PS]-{DNA:(2′-OMe)-RNA} oligomers. RSC Adv 12:26815–26824. https://doi.org/10.1039/d2ra04855h
Jian H, Xu G, Yi Y, Hao Y, Wang Y, **ong L, Wang S, Liu S, Meng C, Wang J, Zhang Y, Chen C, Feng X, Luo H, Zhang H, Zhang X, Wang L, Wang Z, Deng Z, **ao X (2021) The origin and impeded dissemination of the DNA phosphorothioation system in prokaryotes. Nat Commun 12:6382. https://doi.org/10.1038/s41467-021-26636-7
Kaiser S, Byrne SR, Ammann G, Atoi PA, Borland K, Brecheisen R, DeMott MS, Gehrke T, Hagelskamp F, Heiss M, Yoluc Y, Liu L, Zhang Q, Dedon PC, Cao B, Kellner S (2021) Strategies to avoid artifacts in mass spectrometry-based epitranscriptome analyses. Angew Chem Int Ed Engl 60:23885–23893. https://doi.org/10.1002/anie.202106215
Kawaguchi D, Kodama A, Abe N, Takebuchi K, Hashiya F, Tomoike F, Nakamoto K, Kimura Y, Shimizu Y, Abe H (2020) Phosphorothioate modification of mRNA accelerates the rate of translation initiation to provide more efficient protein synthesis. Angew Chem Int Ed Engl 59:17403–17407. https://doi.org/10.1002/anie.202007111
Kellner S, DeMott MS, Cheng CP, Russell BS, Cao B, You D, Dedon PC (2017) Oxidation of phosphorothioate DNA modifications leads to lethal genomic instability. Nat Chem Biol 13:888–894. https://doi.org/10.1038/nchembio.2407
Koziolkiewicz M, Krakowiak A, Kwinkowski M, Boczkowska M, Stec WJ (1995) Stereodifferentiation – the effect of P chirality of oligo (nucleoside phosphorothioates) on the activity of bacterial RNase H. Nucleic Acids Res 23:5000–5005. https://doi.org/10.1093/nar/23.24.5000
Koziołkiewicz M, Wójcik M, Kobylańska A, Karwowski B, Rȩbowska B, Guga P, Stec WJ (1997) Stability of stereoregular oligo(nucleoside phosphorothioate)s in human plasma: diastereoselectivity of plasma 3′-exonuclease. Antisense Nucleic Acid Drug Dev 7:43–48. https://doi.org/10.1089/oli.1.1997.7.43
Koziołkiewicz M, Gendaszewska E, Maszewska M, Stein CA, Stec WJ (2001) The mononucleotide-dependent, non-antisense mechanism of action of phosphodiester and phosphorothioate oligonucleotides depends upon the activity of an ecto-5′-nucleotidase. Blood 98:995–1002. https://doi.org/10.1182/blood.v98.4.995
Krakowiak A, Koziołkiewicz M (1998) Influence of P-chirality of Phosphorothioate oligonucleotides on the activity of AMV-reverse transcriptase. Nucleosides Nucleotides 17:1823–1834. https://doi.org/10.1080/07328319808004720
Krieg AM, Guga P, Stec WJ (2003) P-chirality-dependent immune activation by phosphorothioate CpG oligodeoxynucleotides. Oligonucleotides 13:491–499. https://doi.org/10.1089/154545703322860807
Kulkarni JA, Witzigmann D, Thomson SB et al (2021) The current landscape of nucleic acid therapeutics. Nat Nanotechnol 16:630–643. https://doi.org/10.1038/s41565-021-00898-0
Laurent Q, Martinent R, Moreau D, Winssinger N, Sakai N, Matile S (2021) Oligonucleotide phosphorothioates enter cells by thiol-mediated uptake. Angew Chem Int Ed 60:9102–19106. https://doi.org/10.1002/anie.202107327
Liang JD, Wang ZJ, He XY, Li JL, Zhou XF, Deng ZX (2007) DNA modification by sulfur: analysis of the sequence recognition specificity surrounding the modification sites. Nucleic Acids Res 35:2944–2954. https://doi.org/10.1093/nar/gkm176
Liu G, Fu W, Zhang Z, He Y, Yu H, Wang Y, Wang X, Zhao YL, Deng Z, Wu G, He X (2018) Structural basis for the recognition of sulfur in phosphorothioated DNA. Nat Commun 9:4689. https://doi.org/10.1038/s41467-018-07093-1
Lutz T, Czapinska H, Fomenkov A, Potapov V, Heiter DF, Cao B, Dedon P, Bochtler M, Xu S (2020) Protein domain guided screen for sequence specific and phosphorothioate-dependent restriction endonucleases. Front Microbiol 11:1960. https://doi.org/10.3389/fmicb.2020.01960
Michienzi A, Rossi JJ (2001) Intracellular application of ribozymes. Methods Enzymol 341:581–596. https://doi.org/10.1016/s0076-6879(01)41178-5
Miller CM, Tanowitz M, Donner AJ, Prakash TP, Swayze EE, Harris EN, Seth PP (2018) Receptor-mediated uptake of phosphorothioate antisense oligonucleotides in different cell types of the liver. Nucleic Acid Ther 28:119–127. https://doi.org/10.1089/nat.2017.0709
Nawrot B, Rębowska B, Cieslińska K, Stec WJ (2005) New approach to the synthesis of oligodeoxyribonucleotides modified with phosphorothioates of predetermined sense of P-chirality. Tetrahedron Lett 46:6641–6644. https://doi.org/10.1016/j.tetlet.2005.07.158
Nawrot B, Widera K, Wojcik M, Rebowska B, Goss W, Stec WJ (2007) Map** of the functional phosphate groups in the catalytic core of deoxyribozyme 10-23. FEBS J 274:1062–1072. https://doi.org/10.1111/j.1742-4658.2007.05655.x
Oka N, Wada T, Saigo K (2002) Diastereocontrolled synthesis of dinucleoside phosphorothioates using a novel class of activators, Dialkyl(cyanomethyl)ammonium Tetrafluoroborates. J Am Chem Soc 124:4962–4963. https://doi.org/10.1021/ja017275e
Oka N, Yamamoto M, Sato T, Wada T (2008) Solid-phase synthesis of stereoregular oligodeoxyribonucleoside phosphorothioates using bicyclic oxazaphospholidine derivatives as monomer units. J Am Chem Soc 130:16031–16037. https://doi.org/10.1021/ja805780u
Oka N, Kondo T, Fujiwara S, Maizuru Y, Wada T (2009) Stereocontrolled synthesis of oligoribonucleoside phosphorothioates by an Oxazaphospholidine approach. Org Lett 11:967–970. https://doi.org/10.1021/ol802910k
Østergaard ME, De Hoyos CL, Wan WB, Shen W, Low A, Berdeja A, Vasquez G, Murray S, Migawa MT, Liang XH, Swayze EE, Crooke ST, Seth PP (2020) Understanding the effect of controlling phosphorothioate chirality in the DNA gap on the potency and safety of gapmer antisense oligonucleotides. Nucleic Acids Res 48:1691–1700. https://doi.org/10.1093/nar/gkaa031
Ozga M, Dolot R, Janicka M, Kaczmarek R, Krakowiak A (2010) Histidine triad nucleotide-binding protein 1 (HINT-1) phosphoramidase transforms nucleoside 5′-O-phosphorothioates to nucleoside 5′-O-phosphates. J Biol Chem 285:40809–40818. https://doi.org/10.1074/jbc.M110.162065
Pu T, Liang J, Mei Z, Yang Y, Wang J, Zhang W, Liang WJ, Zhou X, Deng Z, Wang Z (2019) Phosphorothioated DNA is shielded from oxidative damage. Appl Environ Microbiol 85:e00104–e00119. https://doi.org/10.1128/AEM.00104-19
Radzikowska E, Kaczmarek R, Korczyński D, Krakowiak A, Mikołajczyk B, Baraniak J, Guga P, Wheeler KA, Pawlak T, Nawrot B (2020) P-stereocontrolled synthesis of oligo(nucleoside N3’/O5’ phosphoramidothioate)s – opportunities and limitations. RSC Adv 10:35185–35197. https://doi.org/10.1039/d0ra04987e
Saenger W, Hunter WN, Kennard O (1986) DNA conformation is determined by economics in the hydration of phosphate groups. Nature 324:385–388. https://doi.org/10.1038/324385a0
Stec WJ, Grajkowski A, Koziołkiewicz M, Uznański B (1991) Novel route to oligo(deoxyribonucleoside phosphorothioates). Stereocontrolled synthesis of P-chiral oligo(deoxyribonucleoside phosphorothioates). Nucleic Acids Res 19:5883–5888. https://doi.org/10.1093/nar/19.21.5883
Stec WJ, Cierniewski CS, Okruszek A, Kobylańska A, Pawłowska Z, Koziołkiewicz M, Pluskota E, Maciaszek A, Rębowska B, Stasiak M (1997) Stereodependent inhibition of plasminogen activator inhibitor type 1 by Phosphorothioate oligonucleotides: proof of sequence specificity in cell culture and in vivo rat experiments. Antisense Nucleic Drugs Dev 7:567–573. https://doi.org/10.1089/oli.1.1997.7.567
Stec W, Karwowski B, Boczkowska M, Guga P, Koziołkiewicz M, Sochacki M, Wieczorek M, Błaszczyk J (1998) Deoxyribonucleoside 3'-O-(2-Thio- and 2-Oxo-"Spiro"-4,4-Pentamethylene-1,3,2-Oxathiaphospholane)s: monomers for Stereocontrolled synthesis of oligo(deoxyribonucleoside phosphorothioate)s and chimeric PS/PO oligonucleotides. J Am Chem Soc 120:7156–7167. https://doi.org/10.1021/ja973801j
Stein CA, Krieg AM (eds) (1998) Applied antisense oligonucleotide technology. Wiley, New York
Strzelecka D, Smietanski M, Sikorski PJ, Warminski M, Kowalska J, Jemielity J (2020) Phosphodiester modifications in mRNA poly(A) tail prevent deadenylation without compromising protein expression. RNA 26:1815–1837. https://doi.org/10.1261/rna.077099.120
Sun Y, Kong L, Wu G, Cao B, Pang X, Deng Z, Dedon PC, Zhang C, You D (2020) DNA phosphorothioate modifications are widely distributed in the human microbiome. Biomol Ther 10:1175. https://doi.org/10.3390/biom10081175
Sundaralingam M, Pan B (2002) Hydrogen and hydration of DNA and RNA oligonucleotides. Biophys Chem 95:273–282. https://doi.org/10.1016/S0301-4622(01)00262-9
Tomaszewska A, Guga P, Stec WJ (2010) Diastereomerically pure nucleoside-5′-O-(2-thio-4,4-pentamethylene-1,3,2-oxathiaphospholane)s – substrates for synthesis of P-chiral derivatives of nucleoside-5′-O-phosphorothioates. Chirality 23:237–244. https://doi.org/10.1002/chir.20905
Tomaszewska-Antczak A, Jastrzębska K, Maciaszek A, Mikołajczyk B, Guga P (2018) P-Stereodefined phosphorothioate analogs of glycol nucleic acids – synthesis and structural properties. RSC Adv 8:24942–24952. https://doi.org/10.1039/c8ra05568h
Tong T, Chen S, Wang L, Tang Y, Ryu JY, Jiang S, Wu X, Chen C, Luo J, Deng Z, Li Z, Lee SY, Chen S (2018) Occurrence, evolution, and functions of DNA phosphorothioate epigenetics in bacteria. Proc Natl Acad Sci U S A 115:E2988–E2996. https://doi.org/10.1073/pnas.1721916115
Wan WB, Migawa MT, Vasquez G, Murray HM, Nichols JG, Gaus H, Berdeja A, Lee S, Hart CE, Lima WF, Swayze EE, Seth PP (2014) Synthesis, biophysical properties and biological activity of second generation antisense oligonucleotides containing chiral phosphorothioate linkages. Nucleic Acids Res 42:13456–13468. https://doi.org/10.1093/nar/gku1115
Wang L, Chen S, Xu T, Taghizadeh K, Wishnok JS, Zhou X, You D, Deng Z, Dedon PC (2007) Phosphorothioation of DNA in bacteria by dnd genes. Nat Chem Biol 3:709–710. https://doi.org/10.1038/nchembio.2007.39
Wang L, Chen S, Vergin KL, Giovannoni SJ, Chan SW, DeMott MS, Taghizadeh K, Cordero OX, Cutler M, Timberlake S, Alm EJ, Polz MF, Pinhassi J, Deng Z, Dedon PC (2011) DNA phosphorothioation is widespread and quantized in bacterial genomes. Proc Natl Acad Sci U S A 108:2963–2968. https://doi.org/10.1073/pnas.1017261108
Wu Y, Tang Y, Dong X, Zheng YY, Haruehanroengra P, Mao S, Lin Q, Sheng J (2020) RNA phosphorothioate modification in prokaryotes and eukaryotes. ACS Chem Biol 15:1301–1305. https://doi.org/10.1021/acschembio.0c00163
**e X, Liang J, Pu T, Xu F, Yao F, Yang Y, Zhao Y-L, You D, Zhou X, Deng Z, Wang Z (2012) Phosphorothioate DNA as an antioxidant in bacteria. Nucleic Acids Res 40:9115–9124. https://doi.org/10.1093/nar/gks650
**ong W, Zhao G, Yu H, He X (2015) Interactions of Dnd proteins involved in bacterial DNA phosphorothioate modification. Front Microbiol 6:1139. https://doi.org/10.3389/fmicb.2015.01139
**ong L, Liu S, Chen S, **ao Y, Zhu B, Gao Y, Zhang Y, Chen B, Luo J, Deng Z, Chen X, Wang L, Chen S (2019) A new type of DNA phosphorothioation-based antiviral system in archaea. Nat Commun 10:1688. https://doi.org/10.1038/s41467-019-09390-9
**ong X, Wu G, Wei Y, Liu L, Zhang Y, Su R, Jiang X, Li M, Gao H, Tian X, Zhang Y, Hu L, Chen S, Tang Y, Jiang S, Huang R, Li Z, Wang Y, Deng Z, Wang J, Dedon PC, Chen S, Wang L (2020) SspABCD-SspE is a phosphorothioation-sensing bacterial defence system with broad anti-phage activities. Nat Microbiol 5:917–928. https://doi.org/10.1038/s41564-020-0700-6
Xu T, Yao F, Zhou X, Deng Z, You D (2010) A novel host-specific restriction system associated with DNA backbone S-modification in salmonella. Nucleic Acids Res 38:7133–7141. https://doi.org/10.1093/nar/gkq610
Yang Y, Xu G, Liang J, He Y, **ong L, Li H, Bartlett D, Deng Z, Wang Z, **ao X (2017) DNA backbone sulfur-modification expands microbial growth range under multiple stresses by its anti-oxidation function. Sci Rep 7:3516. https://doi.org/10.1038/s41598-017-02445-1
You D, Wang L, Yao F, Zhou X, Deng Z (2007) A novel DNA modification by sulfur: DndA is a NifS-like cysteine desulfurase capable of assembling DndC as an iron-sulfur cluster protein in Streptomyces lividans. Biochemistry 46:6126–6133. https://doi.org/10.1021/bi602615k
Yu CH, Bhattacharya A, Persaud M et al (2021) Nucleic acid binding by SAMHD1 contributes to the antiretroviral activity and is enhanced by the GpsN modification. Nat Commun 12:731. https://doi.org/10.1038/s41467-021-21023-8
Zhou X, He X, Liang J, Li A, Xu T, Kieser T, Helmann JD, Deng Z (2005) A novel DNA modification by sulphur. Mol Microbiol 57:1428–1438. https://doi.org/10.1111/j.1365-2958.2005.04764.x
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Section Editor information
Rights and permissions
Copyright information
© 2023 Springer Nature Singapore Pte Ltd.
About this entry
Cite this entry
Pawłowska, R., Guga, P. (2023). Phosphorothioate Nucleic Acids: Artificial Modification Envisaged by Nature. In: Sugimoto, N. (eds) Handbook of Chemical Biology of Nucleic Acids. Springer, Singapore. https://doi.org/10.1007/978-981-16-1313-5_51-1
Download citation
DOI: https://doi.org/10.1007/978-981-16-1313-5_51-1
Received:
Accepted:
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-1313-5
Online ISBN: 978-981-16-1313-5
eBook Packages: Springer Reference Chemistry and Mat. ScienceReference Module Physical and Materials ScienceReference Module Chemistry, Materials and Physics