Abstract
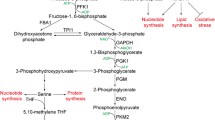
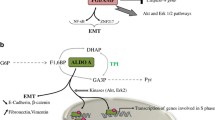
Glycolysis is an ancient metabolic, oxygen-dependent, mostly cytosolic pathway that brings about conversion of glucose to pyruvate with the release of a hydrogen ion and ATP. Reactions in the glycolytic and the pentose phosphate pathways occur in metal-catalyzed, anaerobic Archean oceans, sometimes without enzymes also. Malignant tumor cells undergo glycolysis at ten times the frequency of their normal tissue counterparts. Hypoxia (decreased O2 supply) often occurs in tumor cells, making the cells adapt to this condition by relying on anaerobic glycolysis for ATP, resulting in overexpression of some enzymes in this pathway to generate energy. Factors damaging cells and promoting cancers, such as ROS (reactive oxygen species), are by-products of such inept cellular processes. Most cancers of various origins overexpress enolases, a group of glycolytic enzymes. Enolases primarily catalyze the interconversion of 2-phosphoglycerate and phosphoenolpyruvate in the glycolysis and gluconeogenesis. Similarly, stem cells express increased glycolytic activity; however, they thrive better in reduced ROS levels. In this chapter, we describe the function of enolases and their response to ROS. We also discuss their supplemental role in cancers and stem cells. Glycolysis has been extensively studied in stem cells; however, the role enolases play specifically remains elusive and leaves much to be explored.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Altenberg B, Greenlich KO (2004) Genes of glycolysis are ubiquitously overexpressed in 24 cancer classes. Genomics 84:1014–1020
Balzer E, Moss EG (2007) Localization of the developmental timing regulator Lin28 to mRNP complexes, P-bodies and stress granules. RNA Biol 4:16–25
Bertout JA, Majmundar AJ, Gordan JD, Lam JC, Ditsworth D et al (2009) HIF2α inhibition promotes p53 pathway activity, tumor cell death, and radiation responses. PNAS 106(34):14391–14396
Bigarella CL, Liang R, Ghaffari S (2014) Stem cells and the impact of ROS signalling. Development 141(22):4206–4218
Capello M, Ferri-Borgogno S, Riganti C, Chattaragada MS, Principe M et al (2016) Targeting the Warburg effect in cancer cells through ENO1 knockdown rescues oxidative phosphorylation and induces growth arrest. Oncotarget 7(5):5598–5612
Chang G-C, Liu K-J, Hsieh C-L, Hu TS, Charoenfuprasert S, Liu H-K et al (2006) Identification of α-enolase as an autoantigen in lung cancer: its overexpression is associated with clinical outcomes. Clin Cancer Res 12(19):5746–5754
Cheng C, Long X, Li X, **e M, Guo M (2011) The expression of enolase in the nasopharyngeal cancer tissue. J Clin Otorhinolaryngol Head Neck Surg 25:554–556
Chu PY, Hsu NC, Liao AT, Shih NY, Hou MF, Liu CH (2011) Overexpression of alpha-enolase correlates with poor survival in canine mammary carcinoma. BMC Vet Res 7:62
Comi GP, Fortunato F, Lucchiari S, Bordoni A, Prelle A et al (2001) Beta-enolase deficiency, a new metabolic myopathy of distal glycolysis. Ann Neurol 50(2):202–207
Covello KL, Kehler J, Yu H, Gordan JD, Arsham AM et al (2006) HIF-2α regulates Oct-4: effects of hypoxia on stem cell function, embryonic development, and tumor growth. Genes Dev 20:557–570
Díaz-Ramos A, Roig-Borrellas A, García-Melero A, López-Alemany R (2012) α-Enolase, a multifunctional protein: its role on pathophysiological situations. J Biomed Biotechnol 2012:Article ID 15679514
Dröge W (2002) Aging-related changes in the thiol/disulfide redox state: implications for the use of thiol antioxidants. Exp Gerontol 37:1333–1345
Ejeskär K, Krona C, Carén H et al (2005) Introduction of in vitro transcribed ENO1 mRNA into neuroblastoma cells induces cell death. BMC Cancer 5:161
Feo S, Arcuri D, Piddini E, Passantino R, Giallongo A (2000) ENO1 gene product binds to the c-myc promoter and acts as a transcriptional repressor: relationship with Myc promoter binding protein 1 (MBP-1). FEBS Lett 473(1):47–52
Finkel T (2003) Oxidant signals and oxidative stress. Curr Opin Cell Biol 15:247–254
Fletcher L, Rider CC, Taylor CB (1976) Enolase isoenzymes. III. Biochim Biophys Acta 452(1):245–252
Fougerousse F, Edom-Vovard F, Merkulova T, Ott MO, Durand M, Butler-Browne G, Keller AJ (2001) The muscle-specific enolase is an early marker of human myogenesis. Muscle Res Cell Motil 22(6):535–544
Fu QF, Liu Y, Fan Y, Hua SN, Qu HY, Dong SW et al (2015) Alpha-enolase promotes cell glycolysis, growth, migration and invasion in non-small cell lung cancer through FAK-mediated PI3K/AKT pathway. J Hematol Oncol 8:22
Giallongo A, Venturella S, Oliva D, Barbieri G, Rubino P, Feo S (1993) Structural features of the human gene for muscle-specific enolase. Differential splicing in the 5′-untranslated sequence generates two forms of mRNA. Eur J Biochem 214(2):367–374
Holmström KM, Finkel T (2014) Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat Rev Mol Cell Biol 15:411–421
Iida H, Yahara IJN (1985) Yeast heat-shock protein of Mr 48,000 is an isoprotein of enolase. Nature 315:688–690
Ito K, Suda T (2014) Metabolic requirements for the maintenance of self-renewing stem cells. Nat Rev Mol Cell Biol 15(4):243–256
Janssen-Heininger YMW, Mossman BT, Heintz NH, Forman HJ, Kalyanaraman B, Finkel T et al (2008) Redox-based regulation of signal transduction: principles, pitfalls, and promises. Free Radic Biol Med 45:1–17
Jeffery CJ (1999) Moonlighting proteins. Trends Biochem Sci 24(1):8–11
Ji H, Wang J, Guo J, Li Y, Lian S, Guo W, Yang H, Kong F, Zhen L, Guo L, Liu Y (2016) Progress in the biological function of alpha-enolase. Anim Nutr 2:12–17
Kang HJ et al (2008) A novel role for thioredoxin reductase in the iron metabolism of S. cerevisiae. Biochem Biophys Res Commun 371(1):63–68
Kathagen A et al (2013) Hypoxia and oxygenation induce a metabolic switch between pentose phosphate pathway and glycolysis in glioma stem-like cells. Acta Neuropathol 126:763–780
Kato K, Okagawa Y, Suzuki F, Shimizu A, Mokuno K, Takahashi Y (1983) Immunoassay of human muscle enolase subunit in serum: a novel marker antigen for muscle diseases. Clin Chim Acta 131(1–2):75–85
Lohman K, Meyerhof O (1934) Über die enzymatische umwandlung von phosphoglyzerinsäure in brenztraubensäure und phosphorsäure (enzymatic transformation of phosphoglyceric acid into pyruvic and phosphoric acid). Biochem Z 273:60–72
McAlister L, Holland MJ (1982) Targeted deletion of a yeast enolase structural gene. Identification and isolation of yeast enolase isozymes. J Biol Chem 257(12):7181–7188
Merkulova T, Lucas M, Jabet C, Lamande` N, Rouzeau J-D et al (1997) Biochemical characterization of the mouse muscle-specific enolase: developmental changes in electrophoretic variants and selective binding to other proteins. Biochem J 323(3):791–800
Moore BW, McGregor D (1965) Chromatographic and electrophoretic fractionation of soluble proteins of brain and liver. J Biol Chem 240:1647–1653
Murphy MP (2009) How mitochondria produce reactive oxygen species. Biochem J 417:1–13
Nathan C, Cunningham-Bussel A (2013) Beyond oxidative stress: an immunologist’s guide to reactive oxygen species. Nat Rev Immunol 13:349–361
Pancholi V (2001) Multifunctional alpha-enolase: its role in diseases. Cell Mol Life Sci 58(7):902–920
Peshavaria M, Day IN (1991) Molecular structure of the human muscle-specific enolase gene (ENO3). Biochem J 275(Pt2).
Piast M, Kustrzeba-Wójcicka I, Matusiewicz M, Banaś T (2005) Molecular evolution of enolase. Acta Biochim Pol 52(2):507–513
Principe M, Ceruti P, Shih NY, Chattaragada MS, Rolla S, Conti L, Bestagno M et al (2015) Targeting of surface alpha-enolase inhibits the invasiveness of pancreatic cancer cells. Oncotarget 6(13):11098–11113
Qian X, Xu W, Xu J, Shi Q, Li J, Weng Y, Jiang Z, Feng L, Wang X, Zhou J, ** H (2017) Enolase 1 stimulates glycolysis to promote chemoresistance in gastric cancer. Oncotarget 8:47691–47708
Santana-Rivera Y, Rabelo-Fernández RJ, Quiñones-Díaz BI, Grafals-Ruíz N, Santiago-Sánchez G et al (2020) Reduced expression of enolase-1 correlates with high intracellular glucose levels and increased senescence in cisplatin-resistant ovarian cancer cells. Am J Transl Res 12(4):1275–1292
Sawhney S, Hood K, Shaw A, Braithwaite AW, Stubbs R, Hung WA et al (2015) Alpha-enolase is upregulated on the cell surface and responds to plasminogen activation in mice expressing a Δ133p53α mimic. PLoS One 10:e0116270
Sedoris KC, Thomas SD, Miller DM (2007) C-myc promoter binding protein regulates the cellular response to an altered glucose concentration. Biochemistry 46(29):8659–8668
Song Y, Luo Q, Long H, Hu Z, Que T et al (2014) Alpha-enolase as a potential cancer prognostic marker promotes cell growth, migration, and invasion in glioma. Mol Cancer 13:65
Tahara EB, Navarete FDT, Kowaltowski AJ (2009) Tissue-, substrate-, and site-specific characteristics of mitochondrial reactive oxygen species generation. Free Radic Biol Med 46:1283–1297
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126(4):663–676
Takikita M, Altekruse S, Lynch CF, Goodman MT, Hernandez BY, Green M et al (2009) Associations between selected biomarkers and prognosis in a population-based pancreatic cancer tissue microarray. Cancer Res 69:2950–2955
Vander Heiden MG, Cantley LC, Thompson CB (2009) Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324:1029–1033
Varum S et al (2011) Energy metabolism in human pluripotent stem cells and their differentiated counterparts. PLoS One 6:e20914
Xu C-M, Luo Y-L, Li S, Li Z-X, Jiang L et al (2019) Multifunctional neuron-specific enolase: its role in lung diseases. Biosci Rep 39:427–433
Yan GR, Xu SH, Tan ZL, Yin XF, He QY (2011) Proteomics characterization of gastrokine-1 – induced growth inhibition of gastric cancer cells. Proteomics 11:3657–3664
Young RA, Elliott TJ (1989) Stress proteins, infection, and immune surveillance. Cell 59:5–8
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Singapore Pte Ltd.
About this entry
Cite this entry
Rizwani, W. (2022). Enolase. In: Chakraborti, S., Ray, B.K., Roychoudhury, S. (eds) Handbook of Oxidative Stress in Cancer: Mechanistic Aspects. Springer, Singapore. https://doi.org/10.1007/978-981-15-9411-3_171
Download citation
DOI: https://doi.org/10.1007/978-981-15-9411-3_171
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-9410-6
Online ISBN: 978-981-15-9411-3
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences