Summary
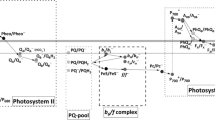
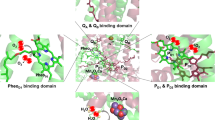
The mechanisms of action of reactive oxygen species (ROS) in plant and animal cells, in chloroplasts, mitochondria, and other sub-cellular compartments such as the endoplasmic reticulum and nucleus, which result in deleterious effects as well as intra-organelle and metabolically significant signaling reactions, are discussed, as well as physiological effects induced by the signaling functions of ROS. The role of ROS in chloroplast retrograde signaling and modulation of abiotic stress response and pathogen defense is discussed. Attention is focused on mechanisms of ROS production, and particularly on the intra-organelle reactions mediated through reduction of oxygen to superoxide in the mitochondrial cytochrome bc 1 and the chloroplast b 6 f complexes, for which understanding of the mechanistic details is facilitated by the existence of high resolution crystal structures. Attention is directed to the structure-based details that underlie the more than tenfold larger specific rate of superoxide production in the b 6 f complex compared to that of the mitochondrial bc 1 complex. Because plastoquinol oxidation by the cytochrome b 6 f complex is known to initiate chloroplast state transitions, the effect of superoxide on the intra-membrane chlorophyll protein kinase that is integral to the cytochrome b 6 f complex, and which underlies the photosynthetic state transitions that regulate the distribution of light energy between the two photosystems, is considered.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Abbreviations
- b p, b n :
-
b-type hemes on electrochemically positive, negative sides of membrane
- cyt:
-
Cytochrome
- DBMIB:
-
2,5-dibromo-3-methyl-6-isopropyl-p-benzoquinone
- Em :
-
midpoint oxidation-reduction potential
- ET:
-
Electron transport
- Fe-S:
-
Iron-sulfur center
- H2O2 :
-
Hydrogen peroxide
- HPV:
-
Hypoxic pulmonary vasoconstriction
- ISP:
-
Rieske iron-sulfur protein
- LHC:
-
Light harvesting complex
- NQNO:
-
2-n-nonyl-4-hydroxyquinoline N-oxide
- n-, p-side:
-
Electrochemically negative-, positive side of membrane
- 1O2 :
-
Singlet oxygen
- \( {{\mathrm{O}}_2}^{\fontsize{3}{5}\selectfont{\underline {\kern0.24em \bullet \kern0.24em }}} \) :
-
Superoxide
- PC:
-
Plastocyanin
- PEWY:
-
Proline-glutamate-tryptophan-tyrosine
- PQ:
-
Plastoquinone
- PQH2 :
-
Plastoquinol
- PQH:
-
Plastosemiquinone
- \( \mathrm{P}{\mathrm{Q}}^{\fontsize{3}{5}\selectfont{\underline {\kern0.24em \bullet \kern0.24em }}} \) :
-
Anionic plastosemiquinone
- PSI, II:
-
Photosystem I, II
- Qp, Qn :
-
Quinone binding site on p-, n-side of membrane
- ROS:
-
Reactive oxygen species
- TDS:
-
Tridecyl-stigmatellin
- UQ:
-
Ubiquinone
- UQH2 :
-
Ubiquinol
- \( \mathrm{U}{{\mathrm{Q}}_2}^{\fontsize{3}{5}\selectfont{\underline {\kern0.24em \bullet \kern0.24em }}} \) :
-
Anionic ubisemiquinone
References
Allen JF (1992) Protein phosphorylation in regulation of photosynthesis. Biochim Biophys Acta 1098:275–335
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Asada K (1999) The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50:601–639
Asada K, Kiso K, Yoshikawa K (1974) Univalent reduction of molecular oxygen by spinach chloroplasts on illumination. J Biol Chem 249:2175–2181
Ashtamker C, Kiss V, Sagi M, Davydov O, Fluhr R (2007) Diverse subcellular locations of cryptogein-induced reactive oxygen species production in tobacco Bright Yellow-2 cells. Plant Physiol 143:1817–1826
Baines CP, Goto M, Downey JM (1997) Oxygen radicals released during ischemic preconditioning contribute to cardioprotection in the rabbit myocardium. J Mol Cell Cardiol 29:207–216
Baker A, Kanofsky JR (1992) Quenching of singlet oxygen by biomolecules from L1210 leukemia cells. Photochem Photobiol 55:523–528
Balazadeh S, Jaspert N, Arif M, Mueller-Roeber B, Maurino VG (2012) Expression of ROS-responsive genes and transcription factors after metabolic formation of H(2)O(2) in chloroplasts. Front Plant Sci 3:234
Baniulis D, Hasan SS, Stofleth JT, Cramer WA (2013) Mechanism of enhanced superoxide production in the cytochrome b(6)f complex of oxygenic photosynthesis. Biochemistry 52:8975–8983
Barajas-Lopez JD, Blanco NE, Strand A (2013) Plastid-to-nucleus communication, signals controlling the running of the plant cell. Biochim Biophys Acta 1833:425–437
Baymann F, Robertson DE, Dutton PL, Mantele W (1999) Electrochemical and spectroscopic investigations of the cytochrome bc1 complex from Rhodobacter capsulatus. Biochemistry 38:13188–13199
Bell EL, Klimova TA, Eisenbart J, Moraes CT, Murphy MP, Budinger GR, Chandel NS (2007) The Qo site of the mitochondrial complex III is required for the transduction of hypoxic signaling via reactive oxygen species production. J Cell Biol 177:1029–1036
Bleier L, Drose S (2013) Superoxide generation by complex III: from mechanistic rationales to functional consequences. Biochim Biophys Acta 1827:1320–1331
Böhme H, Cramer WA (1973) Uncoupler-dependent decrease in midpoint potential of the chloroplast cytochrome b6. Biochim Biophys Acta 325:275–283
Böhme H, Reimer S, Trebst A (1971) Role of plastoquinone in photosynthesis: effect of dibromothymoquinone, an antagonist of plastoquinone, on non cyclic and cyclic electron flow systems in isolated chloroplasts. Z Naturforsch B 26:341–352
Borek A, Sarewicz M, Osyczka A (2008) Movement of the iron-sulfur head domain of cytochrome bc(1) transiently opens the catalytic Q(o) site for reaction with oxygen. Biochemistry 47:12365–12370
Boveris A, Cadenas E, Stoppani AO (1976) Role of ubiquinone in the mitochondrial generation of hydrogen peroxide. Biochem J 156:435–444
Brand MD (2010) The sites and topology of mitochondrial superoxide production. Exp Gerontol 45:466–472
Brandt U, Trumpower B (1994) The protonmotive Q cycle in mitochondria and bacteria. Crit Rev Biochem Mol Biol 29:165–197
Brantmier PJ (2013) Redox regulation of photosynthesis by the cytochrome bf complex: mechanisms and consequences. Thesis/Dissertation, University of Wisconsin Oshkosh. pp 1–151
Brunelle JK, Bell EL, Quesada NM, Vercauteren K, Tiranti V, Zeviani M, Scarpulla RC, Chandel NS (2005) Oxygen sensing requires mitochondrial ROS but not oxidative phosphorylation. Cell Metab 1:409–414
Cadenas E (1995) Mechanisms of oxygen activation and reactive oxygen species detoxification. In: Ahmad S (ed) Oxidative Stress and Antioxidant Defenses in Biology. Springer, New York, pp 1–16
Cadenas E, Boveris A, Ragan CI, Stoppani AO (1977) Production of superoxide radicals and hydrogen peroxide by NADH-ubiquinone reductase and ubiquinol-cytochrome c reductase from beef-heart mitochondria. Arch Biochem Biophys 180:248–257
Cakmak I, Marschner H (1992) Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol 98:1222–1227
Cape JL, Bowman MK, Kramer DM (2006) Understanding the cytochrome bc complexes by what they don’t do. The Q-cycle at 30. Trends Plant Sci 11:46–55
Cape JL, Bowman MK, Kramer DM (2007) A semiquinone intermediate generated at the Qo site of the cytochrome bc1 complex: importance for the Q-cycle and superoxide production. Proc Natl Acad Sci USA 104:7887–7892
Chandel NS (2010) Mitochondrial regulation of oxygen sensing. Adv Exp Med Biol 661:339–354
Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, Schumacker PT (1998) Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Natl Acad Sci USA 95:11715–11720
Chandel NS, McClintock DS, Feliciano CE, Wood TM, Melendez JA, Rodriguez AM, Schumacker PT (2000) Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J Biol Chem 275:25130–25138
Connolly MJ, Aaronson PI (2010) Cell redox state and hypoxic pulmonary vasoconstriction: recent evidence and possible mechanisms. Respir Physiol Neurobiol 174:165–174
Cooley JW, Roberts AG, Bowman MK, Kramer DM, Daldal F (2004) The raised midpoint potential of the [2Fe2S] cluster of cytochrome bc1 is mediated by both the Qo site occupants and the head domain position of the Fe-S protein subunit. Biochemistry 43:2217–2227
Cottage A, Mott E, Wang JH, Sullivan J, MacLean D, Tran L, Choy MK, …, Gray J (2008) GUN1 (GENOMES UNCOUPLED1) Encodes a pentatricopeptide repeat (PPR) protein involved in plastid protein synthesis-responsive retrograde signaling to the nucleus. In: Allen J, Gantt E, Golbeck J, Osmond B (eds) Photosynthesis: Energy From The Sun. Springer, Dordrecht, pp 1201–1205
Covian R, Trumpower BL (2009) The rate-limiting step in the cytochrome bc1 complex is not changed by inhibition of cytochrome b-dependent deprotonation: implications for the mechanism of ubiquinol oxidation at center P of the bc1 complex. J Biol Chem 284:14359–14367
Cramer WA, Baniulis D, Yamashita E, Zhang H, Zatsman AI, Hendrich MP (2008) Cytochrome b6f complex, structure, spectroscopy, and function of the heme cn and n-side electron and proton transfer reactions. In: Fromme P (ed) Photosynthetic Protein Complexes: A Structural Approach. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, pp 155–179
Cramer WA, Hasan SS, Yamashita E (2011) The Q cycle of cytochrome bc complexes: a structure perspective. Biochim Biophys Acta 1807:788–802
Crofts AR (2004) Proton-coupled electron transfer at the Qo-site of the bc1 complex controls the rate of ubihydroquinone oxidation. Biochim Biophys Acta 1655:77–92
Crofts AR, Berry EA (1998) Structure and function of the cytochrome bc1 complex of mitochondria and photosynthetic bacteria. Curr Opin Struct Biol 8:501–509
Crofts AR, Barquera B, Gennis RB, Kuras R, Guergova-Kuras M, Berry EA (1999a) Mechanism of ubiquinol oxidation by the bc(1) complex: different domains of the quinol binding pocket and their role in the mechanism and binding of inhibitors. Biochemistry 38:15807–15826
Crofts AR, Barquera B, Gennis RB, Kuras R, Guergova-Kuras M, Berry EA (1999b) Pathways for proton release during ubihydroquinone oxidation by the bc 1 complex. Proc Natl Acad Sci USA 96:10021–10026
Crofts AR, Hong S, Wilson C, Burton R, Victoria D, Harrison C, Schulten K (2013) The mechanism of ubihydroquinone oxidation at the Qo-site of the cytochrome bc1 complex. Biochim Biophys Acta 1827:1362–1377
Dikanov SA, Samoilova RI, Kolling DR, Holland JT, Crofts AR (2004) Hydrogen bonds involved in binding the Qi-site semiquinone in the bc1 complex, identified through deuterium exchange using pulsed EPR. J Biol Chem 279:15814–15823
Ding H, Moser CC, Robertson DE, Tokito MK, Daldal F, Dutton PL (1995) Ubiquinone pair in the Qo site central to the primary energy conversion reactions of cytochrome bc1 complex. Biochemistry 34:15979–15996
Drose S, Brandt U (2008) The mechanism of mitochondrial superoxide production by the cytochrome bc1 complex. J Biol Chem 283:21649–21654
Drose S, Brandt U (2012) Molecular mechanisms of superoxide production by the mitochondrial respiratory chain. Adv Exp Med Biol 748:145–169
Ershova AN, Popova NV, Berdnikova OS (2011) Production of reactive oxygen species and antioxidant enzymes of pea and soybean plants under hypoxia and high CO2 concentration in medium. Russ J Plant Physiol 58:982–990
Esser L, Quinn B, Li YF, Zhang M, Elberry M, Yu L, Yu CA, **a D (2004) Crystallographic studies of quinol oxidation site inhibitors: a modified classification of inhibitors for the cytochrome bc1 complex. J Mol Biol 341:281–302
Fey V, Wagner R, Brautigam K, Wirtz M, Hell R, Dietzmann A, Leister D, …, Pfannschmidt T (2005) Retrograde plastid redox signals in the expression of nuclear genes for chloroplast proteins of Arabidopsis thaliana. J Biol Chem 280:5318–5328
Forquer I, Covian R, Bowman MK, Trumpower BL, Kramer DM (2006) Similar transition states mediate the Q-cycle and superoxide production by the cytochrome bc1 complex. J Biol Chem 281:38459–38465
Foyer CH, Noctor G (2003) Redox sensing and signalling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria. Physiol Plant 119:355–364
Foyer CH, Shigeoka S (2011) Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol 155:93–100
Foyer CH, Neukermans J, Queval G, Noctor G, Harbinson J (2012) Photosynthetic control of electron transport and the regulation of gene expression. J Exp Bot 63:1637–1661
Furbacher PN, Girvin ME, Cramer WA (1989) On the question of interheme electron transfer in the chloroplast cytochrome b6 in situ. Biochemistry 28:8990–8998
Gadjev I, Vanderauwera S, Gechev TS, Laloi C, Minkov IN, Shulaev V, Apel K, …, Van BF (2006) Transcriptomic footprints disclose specificity of reactive oxygen species signaling in Arabidopsis. Plant Physiol 141:436–445
Galvez-Valdivieso G, Mullineaux PM (2010) The role of reactive oxygen species in signalling from chloroplasts to the nucleus. Physiol Plant 138:430–439
Gechev TS, Gadjev IZ, Hille J (2004) An extensive microarray analysis of AAL-toxin-induced cell death in Arabidopsis thaliana brings new insights into the complexity of programmed cell death in plants. Cell Mol Life Sci 61:1185–1197
Gwak SH, Yang FD, Yu L, Yu CA (1987) Phospholipid-dependent interaction between dibromothymoquinone and iron-sulfur protein in mitochondrial ubiquinol-cytochrome c reductase. Biochim Biophys Acta 890:319–325
Han D, Antunes F, Canali R, Rettori D, Cadenas E (2003) Voltage-dependent anion channels control the release of the superoxide anion from mitochondria to cytosol. J Biol Chem 278:5557–5563
Hasan SS, Cramer WA (2012) Lipid functions in cytochrome bc complexes: an odd evolutionary transition in a membrane protein structure. Philos Trans R Soc B 367:3406–3411
Hasan SS, Yamashita E, Baniulis D, Cramer WA (2013) Quinone-dependent proton transfer pathways in the photosynthetic cytochrome b6f complex. Proc Natl Acad Sci USA 110:4297–4302
Hausenloy DJ, Wynne AM, Yellon DM (2007) Ischemic preconditioning targets the reperfusion phase. Basic Res Cardiol 102:445–452
Hayakawa T, Kanematsu S, Asada K (1984) Occurrence of Cu, Zn-superoxide dismutase in the intrathylakoid space of spinach chloroplasts. Plant Cell Physiol 25:883–889
Heiber I, Stroher E, Raatz B, Busse I, Kahmann U, Bevan MW, Dietz KJ, Baier M (2007) The redox imbalanced mutants of Arabidopsis differentiate signaling pathways for redox regulation of chloroplast antioxidant enzymes. Plant Physiol 143:1774–1788
Henry JP, Juin P, Vallette F, Thieffry M (1996) Characterization and function of the mitochondrial outer membrane peptide-sensitive channel. J Bioenerg Biomembr 28:101–108
Hideg E, Kalai T, Hideg K, Vass I (1998) Photoinhibition of photosynthesis in vivo results in singlet oxygen production detection via nitroxide-induced fluorescence quenching in broad bean leaves. Biochemistry 37:11405–11411
Horn DM (2004) Superoxide production in the cytochrome bf complex of photosynthesis. Thesis/Dissertation, University of Wisconsin-Oshkosh, pp 1–80
Huie RE, Padmaja S (1993) The reaction of no with superoxide. Free Radic Res Commun 18:195–199
Hurt E, Hauska G (1982) Identification of the polypeptides in the cytochrome b6/f complex from spinach chloroplasts with redox-center-carrying subunits. J Bioenerg Biomembr 14:405–424
Inaba T, Yazu F, Ito-Inaba Y, Kakizaki T, Nakayama K (2011) Retrograde signaling pathway from plastid to nucleus. Int Rev Cell Mol Biol 290:167–204
Jiang F, Zhang Y, Dusting GJ (2011) NADPH oxidase-mediated redox signaling: roles in cellular stress response, stress tolerance, and tissue repair. Pharmacol Rev 63:218–242
Jones RW, Whitmarsh J (1988) Inhibition of electron transfer and the electrogenic reaction in the cytochrome bf complex by 2-n-nonyl-4-hydroxyquinolineN-oxide (NQNO) and 2,5-dibromo-3-methyl-6-isopropyl-p-benzoquinone (DBMIB). Biochim Biophys Acta (BBA) Bioenergetics 933:258–268
Junemann S, Heathcote P, Rich PR (1998) On the mechanism of quinol oxidation in the bc1 complex. J Biol Chem 273:21603–21607
Jung HS, Chory J (2010) Signaling between chloroplasts and the nucleus: can a systems biology approach bring clarity to a complex and highly regulated pathway? Plant Physiol 152:453–459
Kallas T (2012) Cytochrome b 6 f complex at the heart of energy transduction and redox signaling. In: Eaton-Rye JJ, Tripathy BC, Sharkey TD (eds) Photosynthesis. Springer, Dordrecht, pp 501–560
Kangasjarvi S, Tikkanen M, Durian G, Aro EM (2014) Photosynthetic light reactions – an adjustable hub in basic production and plant immunity signaling. Plant Physiol Biochem 81:128–134
Karpinski S, Reynolds H, Karpinska B, Wingsle G, Creissen G, Mullineaux P (1999) Systemic signaling and acclimation in response to excess excitation energy in Arabidopsis. Science 284:654–657
Karpinski S, Gabrys H, Mateo A, Karpinska B, Mullineaux PM (2003) Light perception in plant disease defence signalling. Curr Opin Plant Biol 6:390–396
Karpinski S, Szechynska-Hebda M, Wituszynska W, Burdiak P (2013) Light acclimation, retrograde signalling, cell death and immune defences in plants. Plant Cell Environ 36:736–744
Kassner RJ (1972) Effects of nonpolar environments on the redox potentials of heme complexes. Proc Natl Acad Sci U S A 69:2263–2267
Kim C, Meskauskiene R, Apel K, Laloi C (2008) No single way to understand singlet oxygen signalling in plants. EMBO Rep 9:435–439
Kovtun Y, Chiu WL, Tena G, Sheen J (2000) Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants. Proc Natl Acad Sci USA 97:2940–2945
Kurisu G, Zhang H, Smith JL, Cramer WA (2003) Structure of the cytochrome b6f complex of oxygenic photosynthesis: tuning the cavity. Science 302:1009–1014
Kushnareva Y, Murphy AN, Andreyev A (2002) Complex I-mediated reactive oxygen species generation: modulation by cytochrome c and NAD(P) + oxidation-reduction state. Biochem J 368:545–553
Lemeille S, Willig A, Depege-Fargeix N, Delessert C, Bassi R, Rochaix JD (2009) Analysis of the chloroplast protein kinase Stt7 during state transitions. PLoS Biol 7, e45
Lenaz G (1988) Role of mobility of redox components in the inner mitochondrial membrane. J Membr Biol 104:193–209
Li B, Kronzucker HJ, Shi W (2013) Molecular components of stress-responsive plastid retrograde signaling networks and their involvement in ammonium stress. Plant Signal Behav 8, e23107
Liaudet L, Vassalli G, Pacher P (2009) Role of peroxynitrite in the redox regulation of cell signal transduction pathways. Front Biosci (Landmark Ed) 14:4809–4814
Link TA (1997) The role of the ‘Rieske’ iron sulfur protein in the hydroquinone oxidation (Q(P)) site of the cytochrome bc1 complex. The ‘proton-gated affinity change’ mechanism. FEBS Lett 412:257–264
Liu Y, Yang XM, Iliodromitis EK, Kremastinos DT, Dost T, Cohen MV, Downey JM (2008) Redox signaling at reperfusion is required for protection from ischemic preconditioning but not from a direct PKC activator. Basic Res Cardiol 103:54–59
Mansfield KD, Guzy RD, Pan Y, Young RM, Cash TP, Schumacker PT, Simon MC (2005) Mitochondrial dysfunction resulting from loss of cytochrome c impairs cellular oxygen sensing and hypoxic HIF-alpha activation. Cell Metab 1:393–399
Marchi S, Giorgi C, Suski JM, Agnoletto C, Bononi A, Bonora M, De ME, …, Pinton P (2012) Mitochondria-ros crosstalk in the control of cell death and aging. J Signal Transduct 2012:329635
Marino D, Dunand C, Puppo A, Pauly N (2012) A burst of plant NADPH oxidases. Trends Plant Sci 17:9–15
Mehler AH (1951) Studies on reactions of illuminated chloroplasts. I. Mechanism of the reduction of oxygen and other Hill reagents. Arch Biochem Biophys 33:65–77
Mitchell P (1975a) Protonmotive redox mechanism of the cytochrome b-c1 complex in the respiratory chain: protonmotive ubiquinone cycle. FEBS Lett 56:1–6
Mitchell P (1975b) The protonmotive Q cycle: a general formulation. FEBS Lett 59:137–139
Mitchell P (1976) Possible molecular mechanisms of the protonmotive function of cytochrome systems. J Theor Biol 62:327–367
Mittler R, Vanderauwera S, Gollery M, Van BF (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9:490–498
Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti VB, Vandepoele K, Gollery M, …, Van BF (2011) ROS signaling: the new wave? Trends Plant Sci 16:300–309
Miwa S, Muller FL, Beckman KB (2008) The basics of oxidative biochemistry. In: Miwa S, Beckman KB, Muller FL (eds) Oxidative Stress in Aging: From Model Systems to Human Diseases. Humana Press, Totowa, pp 11–35
Moller IM, Jensen PE, Hansson A (2007) Oxidative modifications to cellular components in plants. Annu Rev Plant Biol 58:459–481
Muhlenbock P, Szechynska-Hebda M, Plaszczyca M, Baudo M, Mateo A, Mullineaux PM, Parker JE, …, Karpinski S (2008) Chloroplast signaling and LESION SIMULATING DISEASE1 regulate crosstalk between light acclimation and immunity in Arabidopsis. Plant Cell 20:2339–2356
Mulkidjanian AY (2005) Ubiquinol oxidation in the cytochrome bc1 complex: reaction mechanism and prevention of short-circuiting. Biochim Biophys Acta 1709:5–34
Muller F, Crofts AR, Kramer DM (2002) Multiple Q-cycle bypass reactions at the Qo site of the cytochrome bc1 complex. Biochemistry 41:7866–7874
Muller FL, Roberts AG, Bowman MK, Kramer DM (2003) Architecture of the Qo site of the cytochrome bc1 complex probed by superoxide production. Biochemistry 42:6493–6499
Mullineaux P, Karpinski S (2002) Signal transduction in response to excess light: getting out of the chloroplast. Curr Opin Plant Biol 5:43–48
Nott A, Jung HS, Koussevitzky S, Chory J (2006) Plastid-to-nucleus retrograde signaling. Annu Rev Plant Biol 57:739–759
Ohnishi T, Trumpower BL (1980) Differential effects of antimycin on ubisemiquinone bound in different environments in isolated succinate-cytochrome c reductase complex. J Biol Chem 255:3278–3284
Osyczka A, Moser CC, Dutton PL (2005) Fixing the Q cycle. Trends Biochem Sci 30:176–182
Paddenberg R, Ishaq B, Goldenberg A, Faulhammer P, Rose F, Weissmann N, Braun-Dullaeus RC, Kummer W (2003) Essential role of complex II of the respiratory chain in hypoxia-induced ROS generation in the pulmonary vasculature. Am J Physiol Lung Cell Mol Physiol 284:L710–L719
Penna C, Mancardi D, Rastaldo R, Pagliaro P (2009) Cardioprotection: a radical view free radicals in pre and postconditioning. Biochim Biophys Acta 1787:781–793
Perry AJ, Rimmer KA, Mertens HD, Waller RF, Mulhern TD, Lithgow T, Gooley PR (2008) Structure, topology and function of the translocase of the outer membrane of mitochondria. Plant Physiol Biochem 46:265–274
Pfalz J, Liebers M, Hirth M, Grubler B, Holtzegel U, Schroter Y, Dietzel L, Pfannschmidt T (2012) Environmental control of plant nuclear gene expression by chloroplast redox signals. Front Plant Sci 3:257
Pfannschmidt T (2003) Chloroplast redox signals: how photosynthesis controls its own genes. Trends Plant Sci 8:33–41
Pfannschmidt T, Schutze K, Brost M, Oelmuller R (2001) A novel mechanism of nuclear photosynthesis gene regulation by redox signals from the chloroplast during photosystem stoichiometry adjustment. J Biol Chem 276:36125–36130
Rich PR (2004) The quinone chemistry of bc complexes. Biochim Biophys Acta 1658:165–171
Rich PR, Bendall DS (1980) The redox potentials of the b-type cytochromes of higher plant chloroplasts. Biochim Biophys Acta 591:153–161
Rich PR, Madgwick SA, Moss DA (1991) The interactions of duroquinol, DBMIB and NQNO with the chloroplast cytochrome bf complex. Biochim Biophys Acta (BBA) Bioenergetics 1058:312–328
Rochaix JD (2007) Role of thylakoid protein kinases in photosynthetic acclimation. FEBS Lett 581:2768–2775
Rossel JB, Wilson PB, Hussain D, Woo NS, Gordon MJ, Mewett OP, Howell KA, …, Pogson BJ (2007) Systemic and intracellular responses to photooxidative stress in Arabidopsis. Plant Cell 19:4091–4110
Rutherford AW, Osyczka A, Rappaport F (2012) Back-reactions, short-circuits, leaks and other energy wasteful reactions in biological electron transfer: redox tuning to survive life in O(2). FEBS Lett 586:603–616
Sang M, **e J, Qin XC, Wang WD, Chen XB, Wang KB, Zhang JP, …, Kuang TY (2011) High-light induced superoxide radical formation in cytochrome b(6)f complex from Bryopsis corticulans as detected by EPR spectroscopy. J Photochem Photobiol B 102:177–181
Sarewicz M, Borek A, Cieluch E, Swierczek M, Osyczka A (2010) Discrimination between two possible reaction sequences that create potential risk of generation of deleterious radicals by cytochrome bc(1). Implications for the mechanism of superoxide production. Biochim Biophys Acta 1797:1820–1827
Sasidharan R, Mustroph A (2011) Plant oxygen sensing is mediated by the N-end rule pathway: a milestone in plant anaerobiosis. Plant Cell 23:4173–4183
Schumacker P (2014) Cellular and molecular mechanisms of O2 sensing. In: Swenson ER, Bãrtsch P (eds) High Altitude. Springer, New York, pp 1–22
Shaikhali J, Heiber I, Seidel T, Stroher E, Hiltscher H, Birkmann S, Dietz KJ, Baier M (2008) The redox-sensitive transcription factor Rap2.4a controls nuclear expression of 2-Cys peroxiredoxin A and other chloroplast antioxidant enzymes. BMC Plant Biol 8:48
Shapiguzov A, Vainonen JP, Wrzaczek M, Kangasjarvi J (2012) ROS-talk – how the apoplast, the chloroplast, and the nucleus get the message through. Front Plant Sci 3:292
Sun J, Trumpower BL (2003) Superoxide anion generation by the cytochrome bc1 complex. Arch Biochem Biophys 419:198–206
Suzuki N, Koussevitzky S, Mittler R, Miller G (2012) ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ 35:259–270
Sylvester JT, Shimoda LA, Aaronson PI, Ward JP (2012) Hypoxic pulmonary vasoconstriction. Physiol Rev 92:367–520
Takahashi MA, Asada K (1983) Superoxide anion permeability of phospholipid membranes and chloroplast thylakoids. Arch Biochem Biophys 226:558–566
Telfer A, Bishop SM, Phillips D, Barber J (1994) Isolated photosynthetic reaction center of photosystem II as a sensitizer for the formation of singlet oxygen. Detection and quantum yield determination using a chemical trap** technique. J Biol Chem 269:13244–13253
Tritto I, D’Andrea D, Eramo N, Scognamiglio A, De SC, Violante A, Esposito A, …, Ambrosio G (1997) Oxygen radicals can induce preconditioning in rabbit hearts. Circ Res 80:743–748
Trotta A, Rahikainen M, Konert G, Finazzi G, Kangasjarvi S (2014) Signalling crosstalk in light stress and immune reactions in plants. Philos Trans R Soc Lond B Biol Sci 369:20130235
Trumpower BL (2002) A concerted, alternating sites mechanism of ubiquinol oxidation by the dimeric cytochrome bc(1) complex. Biochim Biophys Acta 1555:166–173
Trumpower BL, Gennis RB (1994) Energy transduction by cytochrome complexes in mitochondrial and bacterial respiration: the enzymology of coupling electron transfer reactions to transmembrane proton translocation. Annu Rev Biochem 63:675–716
Turrens JF, Boveris A (1980) Generation of superoxide anion by the NADH dehydrogenase of bovine heart mitochondria. Biochem J 191:421–427
Turrens JF, Alexandre A, Lehninger AL (1985) Ubisemiquinone is the electron donor for superoxide formation by complex III of heart mitochondria. Arch Biochem Biophys 237:408–414
Vandenabeele S, Vanderauwera S, Vuylsteke M, Rombauts S, Langebartels C, Seidlitz HK, Zabeau M, …, Van BF (2004) Catalase deficiency drastically affects gene expression induced by high light in Arabidopsis thaliana. Plant J 39:45–58
Vanderauwera S, Zimmermann P, Rombauts S, Vandenabeele S, Langebartels C, Gruissem W, Inze D, Van BF (2005) Genome-wide analysis of hydrogen peroxide-regulated gene expression in Arabidopsis reveals a high light-induced transcriptional cluster involved in anthocyanin biosynthesis. Plant Physiol 139:806–821
Vener VA, van Kan PJM, Rich PR, Ohad I, Andersson B (1997) Plastoquinol at the quinol oxidation site of reduced cytochrome bf mediates signal transduction between light and protein phosphorylation: thylakoid protein kinase deactivation by a single-turnover flash. Proc Natl Acad Sci USA 94:1585–1590
Victoria D, Burton R, Crofts AR (2013) Role of the -PEWY-glutamate in catalysis at the Q(o)-site of the Cyt bc(1) complex. Biochim Biophys Acta 1827:365–386
Wang J, Shimoda LA, Sylvester JT (2012) Ca2+ responses of pulmonary arterial myocytes to acute hypoxia require release from ryanodine and inositol trisphosphate receptors in sarcoplasmic reticulum. Am J Physiol Lung Cell Mol Physiol 303:L161–L168
Waypa GB, Schumacker PT (2005) Hypoxic pulmonary vasoconstriction: redox events in oxygen sensing. J Appl Physiol 98:404–414
Waypa GB, Guzy R, Mungai PT, Mack MM, Marks JD, Roe MW, Schumacker PT (2006) Increases in mitochondrial reactive oxygen species trigger hypoxia-induced calcium responses in pulmonary artery smooth muscle cells. Circ Res 99:970–978
Waypa GB, Marks JD, Guzy R, Mungai PT, Schriewer J, Dokic D, Schumacker PT (2010) Hypoxia triggers subcellular compartmental redox signaling in vascular smooth muscle cells. Circ Res 106:526–535
Waypa GB, Marks JD, Guzy RD, Mungai PT, Schriewer JM, Dokic D, Ball MK, Schumacker PT (2013) Superoxide generated at mitochondrial complex III triggers acute responses to hypoxia in the pulmonary circulation. Am J Respir Crit Care Med 187:424–432
Weir EK, Archer SL (1995) The mechanism of acute hypoxic pulmonary vasoconstriction: the tale of two channels. FASEB J 9:183–189
Widger WR, Cramer WA, Herrmann RG, Trebst A (1984) Sequence homology and structural similarity between the b cytochrome of mitochondrial complex III and the chloroplast b6f complex: position of the cytochrome b hemes in the membrane. Proc Natl Acad Sci USA 81:674–678
Wollman FA (2001) State transitions reveal the dynamics and flexibility of the photosynthetic apparatus. EMBO J 20:3623–3630
Wood PM (1988) The potential diagram for oxygen at pH 7. Biochem J 253:287–289
**a D, Esser L, Tang WK, Zhou F, Zhou Y, Yu L, Yu CA (2013) Structural analysis of cytochrome bc1 complexes: implications to the mechanism of function. Biochim Biophys Acta 1827:1278–1294
Yamashita E, Zhang H, Cramer WA (2007) Structure of the cytochrome b6f complex: quinone analogue inhibitors as ligands of heme cn. J Mol Biol 370:39–52
Yin Y, Yang S, Yu L, Yu CA (2010) Reaction mechanism of superoxide generation during ubiquinol oxidation by the cytochrome bc1 complex. J Biol Chem 285:17038–17045
Yu L, Yang S, Yin Y, Cen X, Zhou F, **a D, Yu CA (2009) Chapter 25 Analysis of electron transfer and superoxide generation in the cytochrome bc1 complex. Methods Enzymol 456:459–473
Yurina N, Odintsova M (2011) Plant organelles to nucleus retrograde signaling. In: Shanker A (ed) Abiotic stress response in plants – physiological, biochemical and genetic Perspectives. InTech, Rijeka, pp 55–74
Zatsman AI, Zhang H, Gunderson WA, Cramer WA, Hendrich MP (2006) Heme-heme interactions in the cytochrome b6f complex EPR spectroscopy and correlation with structure. J Am Chem Soc 128:14246–14247
Zhang H, Whitelegge JP, Cramer WA (2001) Ferredoxin:NADP+ oxidoreductase is a subunit of the chloroplast cytochrome b6f complex. J Biol Chem 276:38159–38165
Zhang H, Kurisu G, Smith JL, Cramer WA (2003) A defined protein-detergent-lipid complex for crystallization of integral membrane proteins: the cytochrome b6f complex of oxygenic photosynthesis. Proc Natl Acad Sci U S A 100:5160–5163
Zhang H, Osyczka A, Dutton PL, Moser CC (2007) Exposing the complex III Qo semiquinone radical. Biochim Biophys Acta 1767:883–887
Zito F, Finazzi G, Joliot P, Wollman FA (1998) Glu78, from the conserved PEWY sequence of subunit IV, has a key function in cytochrome b6f turnover. Biochemistry 37:10395–10403
Zurbriggen MD, Carrillo N, Hajirezaei MR (2010) ROS signaling in the hypersensitive response: when, where and what for? Plant Signal Behav 5:393–396
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Baniulis, D., Saif Hasan, S., Miliute, I., Cramer, W.A. (2016). Mechanisms of Superoxide Generation and Signaling in Cytochrome bc Complexes. In: Cramer, W., Kallas, T. (eds) Cytochrome Complexes: Evolution, Structures, Energy Transduction, and Signaling. Advances in Photosynthesis and Respiration, vol 41. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-7481-9_20
Download citation
DOI: https://doi.org/10.1007/978-94-017-7481-9_20
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-017-7479-6
Online ISBN: 978-94-017-7481-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)