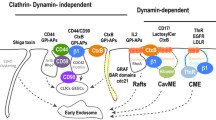
Abstract
Cellular trafficking is essential to maintain critical biological functions. The machinery of proteins and the mechanisms that regulate membrane trafficking is immense and tend to be cell and tissue specific. Mutations in more than 300 genes are known to be associated with disorders of cellular trafficking and include those that affect: (i) membrane trafficking, that mediate the most important pathway to move cargo using membrane bound transport vesicles; (ii) membrane contact sites (MCS) or areas of close apposition between the membranes of organelles; (iii) Other mechanisms such as cytoskeleton mediated cargo/organelle transport, transcytosis, regulation of membrane phospholipids, and gap junctions.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Prinz WA, Toulmay A, Balla T (2020) The functional universe of membrane contact sites. Nat Rev Mol Cell Biol 21(1):7–24
Jackson L (2019) Overview: Traffic at atomic resolution. Traffic 20(12):889
Herrmann JM, Spang A (2015) Intracellular parcel service: current issues in intracellular membrane trafficking. Methods Mol Biol 1270:1–12
De Matteis MA, Luini A (2011) Mendelian disorders of membrane trafficking. N Engl J Med 365(10):927–938
Tokarev A, Alfonso A, Segev N (2000-2013) Overview of intracellular compartments and trafficking pathways. NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health. Madame Curie Bioscience Database (Internet). Austin. Landes Bioscience
Faini M, Beck R, Wieland FT, Briggs JAG (2013) Vesicle coats: structure, function, and general principles of assembly. Trends Cell Biol 23(6):279–288
Passemard S, Perez F, Colin-Lemesre E et al (2017) Golgi trafficking defects in postnatal microcephaly: the evidence for “Golgipathies”. Prog Neurobiol 153:46–63
Wang B, Stanford KR, Kundu M (2020) ER-to-Golgi trafficking and its implication in neurological diseases. Cell 9(2):408
McMahon HT, Boucrot E (2011) Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol 12(8):517–533
Parton RG, Simons K (2007) The multiple faces of caveolae. Nat Rev Mol Cell Biol 8:185–194
Lamaze C, Tardif N, Dewulf M et al (2017) The caveolae dress code: structure and signaling. Curr Opin Cell Biol 47:117–125
Wennerberg K (2005) The Ras superfamily at a glance. J Cell Sci 118:843–846
Morgan NE, Cutrona MB, Simpson JC (2019) Multitasking Rab proteins in autophagy and membrane trafficking: a focus on Rab33b. Int J Mol Sci 20(16):3916
Shi M, Shi C, Xu Y (2017) Rab GTPases: the key players in the molecular pathway of Parkinson’s disease. Front Cell Neurosci 11:81
Sacher M, Shahrzad N, Kamel H et al (2019) TRAPPopathies: an emerging set of disorders linked to variations in the genes encoding transport protein particle (TRAPP)-associated proteins. Traffic 20:5–26
Stanga D, Zhao Q, Milev MP et al (2019) TRAPPC11 functions in autophagy by recruiting ATG28-WIPI4/WDR45 to preautophagosomal membranes. Traffic 20:325–345
Gambardella S, Biagioni F, Ferese R (2016) Vacuolar protein sorting genes in Parkinson’s disease: a re-appraisal of mutations detection rate and neurobiology of disease. Front Neurosci 10:532
Steel D, Zech M, Zhao C (2020) Loss-of-function variants in HOPS complex genes VPS16 and VPS41 cause early onset dystonia associated with lysosomal abnormalities. https://doi.org/10.1002/ana.25879. Online ahead of print
Südhof TC, Rothman JE (2009) Membrane fusion: grappling with SNARE and SM proteins. Science 323:474–477
Anitei M, Hoflack B (2001) Bridging membrane and cytoskeleton dynamics in the secretory and endocytic pathways. Nat Cell Biol 14(1):11–19
Scorrano L, De Matteis MA, Emr S et al (2019) Coming together to define membrane contact sites. Nat Commun 10:1287
** HA, Kraft LM, Chen W et al (2016) Num1 anchors mitochondria to the plasma membrane via two domains with different lipid binding specificities. J Cell Biol 213:513–524
Phillips MJ, Voeltz GK (2016) Structure and function of ER membrane contact sites with other organelles. Nat Rev Mol Cell Biol 17:69–82
Kapogiannis D (2020) Exosome biomarkers revolutionize preclinical diagnosis of neurodegenerative diseases and assessment of treatment responses in clinical trials. Adv Exp Med Biol 1195:149
Pulgar VM (2019) Transcytosis to cross the blood brain barrier, new advancements and challenges. Front Neurosci 12:1019
Graap M, Wrede A, Schweizer M et al (2013) Choroid plexus transcytosis and exosome shuttling deliver folate into brain parenchyma. Nat Commun 4:2123
Gantzel RH, Mogensen LS, Mikkelsen SA et al (2017) Disease mutation reveal residues critical to the interaction of P4-ATPases with lipid substrates. Sci Rep 7(1):10418
Giaume CB, Naus CC, Saez JC, Leybaert L (2021) Glial connexins and pannexins in the healthy and diseased brain. Physiol Rev 101(1):93–145
García-Cazorla A, Saudubray JM (2018) Cellular neurometabolism: a tentative to connect cell biology and metabolism in neurology. J Inherit Metab Dis 41(6):1043–1054
Wojnacki J, Galli T (2016) Membrane traffic during axon development. Dev Neurobiol 76(11):1185–1200
Lalani SR, Liu P, Rosenfeld JA et al (2016) Recurrent muscle weakness with rhabdomyolysis, metabolic crises, and cardiac arrhythmia due to bi-allelic TANGO2 mutations. Am J Hum Genet 98:347–357
Kremer LS, Distelmaier F, Alhaddad B et al (2016) Bi-allelic truncating mutations in TANGO2 cause infancy-onset recurrent metabolic crises with encephalocardiomyopathy. Am J Hum Genet 98:58–362
Bérat CM, Montealegre S, Wiedemann A (2020) Clinical and biological characterization of 20 patients with TANGO2 deficiency indicates novel triggers of metabolic crises and no primary energetic defect. J Inherit Metab Dis. https://doi.org/10.1002/jimd.12314. Online ahead of print
Covone AE, Fiorillo C, Acquaviva M et al (2016) WES in a family trio suggests involvement of TECPR2 in a complex form of progressive motor neuron disease. Clin Genet 90(2):182–185
Khani M, Taheri H, Shamshiri H et al (2019) Continuum of phenotypes in hereditary motor and sensory neuropathy with proximal predominance and Charcot-Marie-Tooth patients with TFG mutation. Am J Med Genet A 179(8):1507–1515
Paloneva J, Manninen T, Christman G (2002) Mutations in two genes encoding different subunits of a receptor signaling complex result in an identical disease phenotype. Am J Hum Genet 71:656–662
Cacciagli P, Desvignes JP, Girard N et al (2014) AP1S2 is mutated in X-linked Dandy-Walker malformation with intellectual disability, basal ganglia disease and seizures (Pettigrew syndrome). Europ J Hum Genet 22:363–368
Sanger A, Hirst J, Davies AK, Robinson MS (2019) Adaptor protein complexes and disease at a glance. J Cell Sci 132(20):jcs222992
Han C, Alkhater R, Froukh T et al (2016) Epileptic encephalopathy caused by mutations in the guanine nucleotide exchange factor DENND5A. Am J Hum Genet 99:1359–1367
Banne E, Atawneh O, Henneke M et al (2013) West syndrome, microcephaly, grey matter heterotopia and hypoplasia of corpus callosum due to a novel ARFGEF2 mutation. J Med Genet 50:772–775
Gupta HV, Vengoechea J, Sahaya K, Virmani T (2015) A splice site mutation in ATP6AP2 causes X-linked intellectual disability, epilepsy, and parkinsonism. Parkinsonism Relat Disord 21:1473–1475
Chai M, Su L, Hao X et al (2018) Identification of genes and signaling pathways associated with arthrogryposis-renal dysfunction-cholestasis syndrome using weighted correlation network analysis. Int J Mol Med 42(4):2238–2246
Liu H, Wu C (2017) Charcot Marie tooth 2B peripheral sensory neuropathy: how Rab7 mutations impact NGF signalling. Int J Mol Sci 18(2):324
Cullup T, Kho AL, Dionisi-Vici C et al (2013) Recessive mutations in EPG5 cause Vici syndrome, a multisystem disorder with defective autophagy. Nat Genet 45(1):83–87
Zatyka M, Sarkar S, Barrett T (2020) Autophagy in rare (NonLysosomal) neurodegenerative diseases. J Mol Biol 432(8):2735–2753
Sleigh JN, Tossor AM, Fellows AD et al (2019) Axonal transport and neurological disease. Nat Rev Neurol 15(12):691–703
Namba T, Funahashi Y, Nakamuta S et al (2015) Extracellular and intracellular signaling for neuronal polarity. Physiol Rev 95:995–1024
Cortès-Saladelafont E, Lipstein N, García-Cazorla À (2018) Presynaptic disorders: a clinical and pathophysiological approach focused on the synaptic vesicle. J Inherit Metab Dis 41(6):1131–1145
Radler MR, Suber A, Spiliotis ET (2020) Spatial control of membrane traffic in neuronal dendrites. Mol Cell Neurosci 105:103492
Yarwood R, Hellicar J, Woodman PG, Lowe M (2020) Membrane trafficking in health and disease. Dis Model Mech 13(4):dmm043448
Mutoh H, Kato M, Akita T et al (2018) Biallelic variants in CNPY3, encoding an endoplasmic reticulum chaperone, cause early-onset epileptic encephalopathy. Am J Hum Genet 102:321–329
Olson HE, Jean-Marcais N, Yang E et al (2018) PACS2heterozygous missense variant causes neonatal-onset developmental epileptic encephalopathy, facial dysmorphism, and cerebellar dysgenesis. Am J Hum Genet 102:995–1007. Note: Erratum: Am. J. Hum. Genet. 103: 631 only
Duis J, Dean S, Applegate C et al (2016) KIF5A mutations cause an infantile onset phenotype including severe myoclonus with evidence of mitochondrial dysfunction. Ann Neurol 80:633–637
Alcantara D, O'Driscoll M (2014) Congenital microcephaly. Am J Med Genet C Semin Med Genet 166C(2):124–139
Aligianis IA, Johnson CA, Gissen P et al (2005) Mutations of the catalytic subunit ofRAB3GAP cause Warburg Micro syndrome. Nat Genet 37:221–223
Stanga D, Qingchuan A, Milev MP (2019) TRAPPC11 functions in autophagy by recruiting ATG2B-WIPI4/WDR45 to preautophagosomal membranes. Traffic 20(5):325–345
Sferra A, Petrini S, Bellacchio E et al (2020) TUBB variants underlying different phenotypes result in altered vesicle trafficking and microtubule dynamics. Int J Mol Sci 21(4):1385
Aggarwal S, Bhowmik AD, Ramprasad VL et al (2016) A splice site mutation in HERC1 leads to syndromic intellectual disability with macrocephaly and facial dysmorphism: further delineation of the phenotypic spectrum. Am J Med Genet 170A:1868–1873
Reijnders MRF, Ansor NM, Kousi M et al (2017) RAC1 missense mutations in developmental disorders with diverse phenotypes. Am J Hum Genet 101:466–477
Edvardson S, Ashikov A, Jalas C et al (2013) Mutations in SLC35A3cause autism spectrum disorder, epilepsy and arthrogryposis. J Med Genet 50:733–739
Reinstein E, Drasinover V, Lotan R, Gal-Tanamy M, Nachman IB, Eyal E, Jaber L, Magal N, Shohat M et al (2018) Mutations in ERGIC1 cause arthrogryposis multiplex congenita, neuropathic type. Clin Genet 93:160–163
Gueneau L, Fish RJ, Shamseldin HE et al (2018) KIAA1109 variants are associated with a severe disorder of brain development and arthrogryposis. Am J Hum Genet 102:116–132
Smith H, Galmes R, Gogolina E et al (2012) Associations among genotype, clinical phenotype, and intracellular localization of trafficking proteins in ARC syndrome. Hum Mutat 33:1656–1664
Bogershausen N, Shahrzad N, Chong JX et al (2013) Recessive TRAPPC11 mutations cause a disease spectrum of limb girdle muscular dystrophy and myopathy with movement disorder and intellectual disability. Am J Hum Genet 93:181–190
Garbern JY, Neumann M, Trojanowski JQ et al (2010) A mutation affecting the sodium/proton exchanger, SLC9A6, causes mental retardation with tau deposition. Brain 133:1391–1402
Martinelli S, Krumbach OHF, Pantaleoni F et al (2018) Functional dysregulation of CDC42 causesdiverse developmental phenotypes. Am J Hum Genet 102:309–320
Tessa A, Battini R, Rubegni A et al (2016) Identification of mutations in AP4S1/SPG52 through next generation sequencing in three families. Eur J Neurol 23(10):1580–1587
Zivony-Elboum Y, Westbroek W, Kfir N (2012) A founder mutation in Vps37A causes autosomal recessive complex hereditary spastic paraparesis. J Med Genet 49(7):462–472
Bandres-Ciga S, Saez-Atienzar S, Bonet-Ponce L (2019) The endocytic membrane trafficking pathway plays a major role in the risk of Parkinson’s disease. Mov Disord 34(4):460–468
Giannandrea M, Bianchi V, Mignogna ML et al (2010) Mutations in the small GTPase gene RAB39B are responsible for X-linked mental retardation associated with autism, epilepsy, and macrocephaly. Am J Hum Genet 86:185–195
Peeters K, Litvinenko I, Asselbergh B et al (2013) Molecular defects in the motor adaptor BICD2 cause proximal spinal muscular atrophy with autosomal-dominant inheritance. Am J Hum Genet 92(6):955–964
Kurth I, Pamminger T, Hennings JC et al (2009) Mutations in FAM134B, encoding a newly identified Golgi protein, cause severe sensory and autonomic neuropathy. Nat Genet 41:1179–1181
Guerreiro R, Wojtas A, Bras J et al (2013) TREM2 variants in Alzheimer’s disease. N Eng J Med 368:117–127
Piano Mortari E, Folgiero V, Marcellini V (2018) The Vici syndrome protein EPG5 regulates intracellular nucleic acid trafficking linking autophagy to innate and adaptive immunity. Autophagy 14(1):22–37
Ajitkumar A, Yarrarapu SNS, Ramphul K (2020) Chediak Higashi Syndrome. 2020 Aug 13. In: StatPearls [Internet]. StatPearls Publishing, Treasure Island (FL). PMID: 29939658
De Jesus RW, Young LR (2020) Hermansky-Pudlak syndrome. Semin Respir Crit Care Med 41(2):238–246
Fuchs-Telem D, Stewart H, Rapaport D et al (2011) CEDNIK syndrome results from loss-of-function mutations in SNAP29. Br J Dermatol 164:610–616
Cohn DH, Ehtesham N, Krakow D et al (2003) Mental retardation and abnormal skeletal development (Dyggve-Melchior-Clausen dysplasia) due to mutations in a novel, evolutionarily conserved gene. Am J Hum Genet 72:419–428
Staufner C, Peters B, Wagner M et al (2020) Defining clinical subgroups and genotype-phenotype correlations in NBAS-associated disease across 110 patients. Genet Med 22(3):610–621
Schmidt WM, Kraus C, Hoger H et al (2007) Mutation in the Scyl1 gene encoding amino-terminal kinase-like protein causes a recessive form of spinocerebellar neurodegeneration. EMBO Rep 8:691–697
Lewis RA, Nussbaum RL, Brewer ED (2001) Lowe syndrome. Jul 24 [updated 2019 Apr 18]. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, LJH B, Stephens K, Amemiya A (eds) GeneReviews® [Internet]. University of Washington, Seattle; 1993–2020, Seattle
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer-Verlag GmbH Germany, part of Springer Nature
About this chapter
Cite this chapter
García-Cazorla, Á., Dionisi-Vici, C., Saudubray, JM. (2022). Disorders of Cellular Trafficking. In: Saudubray, JM., Baumgartner, M.R., García-Cazorla, Á., Walter, J. (eds) Inborn Metabolic Diseases. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-63123-2_44
Download citation
DOI: https://doi.org/10.1007/978-3-662-63123-2_44
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-63122-5
Online ISBN: 978-3-662-63123-2
eBook Packages: MedicineMedicine (R0)