Abstract
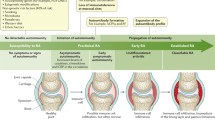
Rheumatoid arthritis (RA) is almost invariably associated with significant symptomatology. In the early stages of the disease, joint pain and stiffness are the dominant symptoms, but patients also frequently experience general symptoms due to the systemic inflammatory state. Extreme fatigue and lassitude, and even slight fever and profound weight loss are not unusual at this stage. The musculoskeletal symptoms may already in the earlier phase engender significant functional impairment and restriction of activities which are, however, still reversible. At later stages of the disease, inflammatory symptoms may continue to be severe but in contrast to more benign musculoskeletal conditions RA has the potential to cause severe and irreversible damage to the anatomical structures of the joints as well. Thus, erosions and other damage to the bony surfaces of the joints, and cartilage break-down are hallmarks of the disease that when advanced are easily recognized on plain radiographs, but that may at even earlier stages be detected through more sensitive imaging techniques such as magnetic resonance imaging (MRI) and ultrasound.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Fuchs HA, Kaye JJ, Callahan LF, Nance EP, Pincus T. Evidence of significant radiographic damage in rheumatoid arthritis within the first 2 years of disease. J Rheumatol. 1989;16:585-591.
Wick MC, Lindblad S, Weiss RJ, Klareskog L, van Vollenhoven RF. Clinical and radiological disease-course in a Swedish DMARD-treated early RA-inception cohort: an observational study. Scand J Rheumatol. 2004;33:380-384.
Wick MC, Lindblad S, Weiss RJ, Klareskog L, van Vollenhoven RF. Estimated prediagnosis radiological progression: an important tool for studying the effects of early disease modifying antirheumatic drug treatment in rheumatoid arthritis. Ann Rheum Dis. 2005;64:134-137.
de Haas WH, de Boer W, Griffioen F, Oosten-Elst P. Rheumatoid arthritis, typus robustus. Ann Rheum Dis. 1973;32:91-92.
Fries JF. The assessment of disability: from first to future principles. Br J Rheumatol. 1983;22:48-58.
Watson DJ, Rhodes T, Guess HA. All-cause mortality and vascular events among patients with rheumatoid arthritis, osteoarthritis, or no arthritis in the UK General Practice Research Database. J Rheumatol. 2003;30:1196-1202.
Wallberg-Jonsson S, Johansson H, Ohman ML, Rantapaa-Dahlqvist S. Extent of inflammation predicts cardiovascular disease and overall mortality in seropositive rheumatoid arthritis. A retrospective cohort study from disease onset. J Rheumatol. 1999;26:2562-2571.
Askling J. Malignancy and rheumatoid arthritis. Curr Rheumatol Rep. 2007;9:421-426.
Strangfeld A, Eveslage M, Schneider M, et al. Treatment benefit or survival of the fittest: what drives the time-dependent decrease in serious infection rates under TNF inhibition and what does this imply for the individual patient? Ann Rheum Dis. 2011;70:1914-1920.
van der Heijde DM, van ‘t Hof MA, van Riel PL, et al. Judging disease activity in clinical practice in rheumatoid arthritis: first step in the development of a disease activity score. Ann Rheum Dis. 1990;49:916-920.
Prevoo ML, van ‘t Hof MA, Kuper HH, et al. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44-48.
Wells G, Becker JC, Teng J, et al. Validation of the 28-joint Disease Activity Score (DAS28) and European League Against Rheumatism response criteria based on C-reactive protein against disease progression in patients with rheumatoid arthritis, and comparison with the DAS28 based on erythrocyte sedimentation rate. Ann Rheum Dis. 2009;68:954-960.
Fransen J, van Riel PL. The Disease Activity Score and the EULAR response criteria. Clin Exp Rheumatol. 2005;23:S93-S99.
Smolen JS, Breedveld FC, Schiff MH, et al. A simplified disease activity index for rheumatoid arthritis for use in clinical practice. Rheumatology (Oxford). 2003;42:244-257.
Aletaha D, Nell VP, Stamm T, et al. Acute phase reactants add little to composite disease activity indices for rheumatoid arthritis: validation of a clinical activity score. Arthritis Res Ther. 2005;7:R796-R806.
Stucki G, Liang MH, Stucki S, Bruhlmann P, Michel BA. A self-administered rheumatoid arthritis disease activity index (RADAI) for epidemiologic research. Psychometric properties and correlation with parameters of disease activity. Arthritis Rheum. 1995;38:795-798.
Pincus T, Yazici Y, Bergman M, Swearingen C, Harrington T. A proposed approach to recognise “near-remission” quantitatively without formal joint counts or laboratory tests: a patient self-report questionnaire routine assessment of patient index data (RAPID) score as a guide to a “continuous quality improvement” s. Clin Exp Rheumatol. 2006;24:S60-S73.
Fries JF, Spitz PW, Young DY. The dimensions of health outcomes: the health assessment questionnaire, disability and pain scales. J Rheumatol. 1982;9:789-793.
Bruce B, Fries JF. The Stanford Health Assessment Questionnaire: a review of its history, issues, progress, and documentation. J Rheumatol. 2003;30:167-178.
Wolfe F. Why the HAQ-II can be an effective substitute for the HAQ. Clin Exp Rheumatol. 2005;23:S29-S30.
Pincus T, Summey JA, Soraci SA, Jr., Wallston KA, Hummon NP. Assessment of patient satisfaction in activities of daily living using a modified Stanford Health Assessment Questionnaire. Arthritis Rheum. 1983;26:1346-1353.
Weinberger M, Samsa GP, Hanlon JT, et al. An evaluation of a brief health status measure in elderly veterans. J Am Geriatr Soc. 1991;39:691-694.
EuroQol Group. EuroQol–a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199-208.
Fries JF, Witter J, Rose M, Cella D, Khanna D, Morgan-DeWitt E. Item response theory, computerized adaptive testing, and PROMIS: assessment of physical function. J Rheumatol. 2014;41:153-158.
Rose M, Bjorner JB, Becker J, Fries JF, Ware JE. Evaluation of a preliminary physical function item bank supported the expected advantages of the Patient-Reported Outcomes Measurement Information System (PROMIS). J Clin Epidemiol. 2008;61:17-33.
Rose M, Bjorner JB, Gandek B, Bruce B, Fries JF, Ware JE, Jr. The PROMIS Physical Function item bank was calibrated to a standardized metric and shown to improve measurement efficiency. J Clin Epidemiol. 2014;67:516-526.
Felson DT, Anderson JJ, Boers M, et al. The American College of Rheumatology preliminary core set of disease activity measures for rheumatoid arthritis clinical trials. The Committee on Outcome Measures in Rheumatoid Arthritis Clinical Trials. Arthritis Rheum. 1993;36:729-740.
Felson DT, Anderson JJ, Boers M, et al. American College of Rheumatology. Preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum. 1995;38:727-735.
van Gestel AM, Prevoo ML, van ‘t Hof MA, van Rijswijk MH, van de Putte LB, van Riel PL. Development and validation of the European League Against Rheumatism response criteria for rheumatoid arthritis. Comparison with the preliminary American College of Rheumatology and the World Health Organization/International League Against Rheumatism Criteria. Arthritis Rheum. 1996;39:34-40.
Smolen JS, Aletaha D, Bijlsma JW, et al. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis. 2010;69:631-637.
Felson DT, Smolen JS, Wells G, et al. American College of Rheumatology/European League against Rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials. Ann Rheum Dis. 2011;70:404-413.
Masri KR, Shaver TS, Shahouri SH, et al. Validity and reliability problems with patient global as a component of the ACR/EULAR remission criteria as used in clinical practice. J Rheumatol. 2012;39:1139-1145.
Wilske KR, Healey LA. Remodeling the pyramid–a concept whose time has come. J Rheumatol. 1989;16:565-567.
Gremillion RB, van Vollenhoven RF. Rheumatoid arthritis. Designing and implementing a treatment plan. Postgrad Med. 1998;103:118.
O’Dell JR, Haire CE, Erikson N, et al. Treatment of rheumatoid arthritis with methotrexate alone, sulfasalazine and hydroxychloroquine, or a combination of all three medications. N Engl J Med. 1996;334:1287-1291.
Tugwell P, Pincus T, Yocum D, et al. Combination therapy with cyclosporine and methotrexate in severe rheumatoid arthritis. The Methotrexate-Cyclosporine Combination Study Group. N Engl J Med. 1995;333:137-141.
Willkens RF, Urowitz MB, Stablein DM, et al. Comparison of azathioprine, methotrexate, and the combination of both in the treatment of rheumatoid arthritis. A controlled clinical trial. Arthritis Rheum. 1992;35:849-856.
McCarty DJ, Carrera GF. Intractable rheumatoid arthritis. Treatment with combined cyclophosphamide, azathioprine, and hydroxychloroquine. JAMA. 1982;248:1718-1723.
Walters MT, Cawley MI. Combined suppressive drug treatment in severe refractory rheumatoid disease: an analysis of the relative effects of parenteral methylprednisolone, cyclophosphamide, and sodium aurothiomalate. Ann Rheum Dis. 1988;47:924-929.
Wollenhaupt J, Zeidler H. Combined administration of long-acting antirheumatic drugs in the treatment of chronic polyarthritis. Dtsch Med Wochenschr. 1997;122:1219-1223.
Kremer JM, Genovese MC, Cannon GW, et al. Concomitant leflunomide therapy in patients with active rheumatoid arthritis despite stable doses of methotrexate. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2002;137:726-733.
Weinblatt ME, Dixon JA, Falchuk KR. Serious liver disease in a patient receiving methotrexate and leflunomide. Arthritis Rheum. 2000;43:2609-2011.
Svensson B, Boonen A, Albertsson K, van der Heijde D, Keller C, Hafstrom I. Low-dose prednisolone in addition to the initial disease-modifying antirheumatic drug in patients with early active rheumatoid arthritis reduces joint destruction and increases the remission rate: a two-year randomized trial. Arthritis Rheum. 2005;52:3360-3370.
Wassenberg S, Rau R, Steinfeld P, Zeidler H. Very low-dose prednisolone in early rheumatoid arthritis retards radiographic progression over two years: a multicenter, double-blind, placebo-controlled trial. Arthritis Rheum. 2005;52:3371-3380.
Hetland ML, Stengaard-Pedersen K, Junker P, et al. Aggressive combination therapy with intra-articular glucocorticoid injections and conventional disease-modifying anti-rheumatic drugs in early rheumatoid arthritis: second-year clinical and radiographic results from the CIMESTRA study. Ann Rheum Dis. 2008;67:815-822.
Horslev-Petersen K, Hetland ML, Junker P, et al. Adalimumab added to a treat-to-target strategy with methotrexate and intra-articular triamcinolone in early rheumatoid arthritis increased remission rates, function and quality of life. The OPERA Study: an investigatorinitiated, randomised, double-blind, parallel-group, placebo-controlled trial. Ann Rheum Dis. 2014;73:654-661.
Cronstein BN, Eberle MA, Gruber HE, Levin RI. Methotrexate inhibits neutrophil function by stimulating adenosine release from connective tissue cells. Proc Natl Acad Sci USA. 1991;88:2441-2445.
Cronstein BN, Naime D, Ostad E. The antiinflammatory mechanism of methotrexate. Increased adenosine release at inflamed sites diminishes leukocyte accumulation in an in vivo model of inflammation. J Clin Invest. 1993;92:2675-2682.
Cronstein BN, Naime D, Ostad E. The antiinflammatory effects of methotrexate are mediated by adenosine. Adv Exp Med Biol. 1994;370:411-416.
O’Dell JR, Leff R, Paulsen G, Haire C, Mallek J, Eckhoff PJ, et al. Treatment of rheumatoid arthritis with methotrexate and hydroxychloroquine, methotrexate and sulfasalazine, or a combination of the three medications: results of a two-year, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2002;46:1164-1170.
Weinblatt ME, Kremer JM, Coblyn JS, et al. Pharmacokinetics, safety, and efficacy of combination treatment with methotrexate and leflunomide in patients with active rheumatoid arthritis. Arthritis Rheum. 1999;42:1322-1328.
SVT Bild. Nanna Svartz. http://www.svtbild.se/. Accessed October 12, 2015.
Stenstrom CH, Minor MA. Evidence for the benefit of aerobic and strengthening exercise in rheumatoid arthritis. Arthritis Rheum. 2003;49:428-434.
Forseth KO, Hafstrom I, Husby G, Opava C. Comprehensive rehabilitation of patients with rheumatic diseases in a warm climate: a literature review. J Rehabil Med. 2010;42:897-902.
Hafstrom I, Ringertz B, Spangberg A, et al. A vegan diet free of gluten improves the signs and symptoms of rheumatoid arthritis: the effects on arthritis correlate with a reduction in antibodies to food antigens. Rheumatology (Oxford). 2001;40:1175-1179.
Skoldstam L, Hagfors L, Johansson G. An experimental study of a Mediterranean diet intervention for patients with rheumatoid arthritis. Ann Rheum Dis. 2003;62:208-214.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
van Vollenhoven, R.F. (2016). General Treatment Aspects. In: Biologics for the Treatment of Rheumatoid Arthritis. Adis, Cham. https://doi.org/10.1007/978-3-319-13108-5_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-13108-5_2
Published:
Publisher Name: Adis, Cham
Print ISBN: 978-3-319-13107-8
Online ISBN: 978-3-319-13108-5
eBook Packages: MedicineMedicine (R0)