Abstract
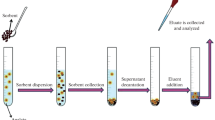
Matrix solid phase dispersion (MSPD) is an extremely simple, fast and effective technique of sample preparation, in which sample destruction, homogenization and extraction takes place simultaneously in a single step of the MSPD procedure. This technique has been known for nearly 30 years. At that time, new types of sorbents, abrasive materials and extraction fluids were introduced to the MSPD procedure, and the process itself began to be assisted by ultrasounds, vortexing or microwaves. The result of these improvements is far-reaching miniaturization accompanied by greater isolation efficiency per unit of time achieved using more ecological and economical analytical procedures. Due to its flexibility and versatility, the MSPD technique is currently being implemented in various research laboratories for the isolation of endo- and exogenous compounds, including hazardous or prohibited compounds, volatile and non-volatile, present in various concentrations not only in solid but also in semi-solid and viscous samples, which can be generally grouped into environmental, biological, pharmaceuticals, food and everyday products samples. This chapter outlines the various analytical challenges where MSPD is useful and the sorbents that are currently being used to meet these challenges, with particular emphasis on new research areas where the MSPD process has come into use.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Abbreviations
- β-CD:
-
β-Cyclodextrin
- C18:
-
Chemically bound octadecyl phase
- CDs:
-
Cyclodextrins
- CLC:
-
Chiral liquid chromatography
- DESs:
-
Deep eutectic solvents
- DLLME:
-
Dispersive liquid–liquid microextraction
- EDCs:
-
Endocrine disrupting chemicals
- EOs:
-
Essential oils
- FID:
-
Flame ionization detector
- GC:
-
Gas chromatography
- GCB:
-
Graphitized carbon black
- HLLME:
-
Homogeneous liquid–liquid microextraction
- HPLC:
-
High performance liquid chromatography
- ILs:
-
Ionic liquids
- IL-VF-MSPD:
-
Ionic liquid based vortex-forced matrix solid phase dispersion
- IP-SPE:
-
Ion pair—solid-phase extraction
- LC:
-
Liquid chromatography
- LLE:
-
Liquid–liquid extraction
- LOD:
-
Limit of detection
- LOQ:
-
Limit of quantification
- MALDI:
-
Matrix assisted laser desorption ionization
- MA-MSPD:
-
Microwave-assisted matrix solid phase extraction
- MCs:
-
Microcystins
- MEEKC:
-
Microemulsion electrokinetic chromatography
- MEPS:
-
Microextraction in packed syringe or microextraction by packed sorbent
- MI:
-
Molecularly imprinted
- MIM:
-
Molecularly imprinted microsphere
- MI-MSPD:
-
Molecularly imprinted matrix solid-phase dispersion
- MIPs:
-
Molecularly-imprinted polymers
- MOF:
-
Metal–organic framework
- MS:
-
Mass spectrometry
- MSPD:
-
Matrix solid-phase dispersion
- NMR:
-
Nuclear Magnetic Resonance
- NP:
-
Normal phase
- PLE:
-
Pressurized liquid extraction
- PSA:
-
Primary and secondary amine
- Q:
-
Quadrupole
- QuECHERS:
-
Quick Easy Cheap Effective Raged and Safe
- RP:
-
Reversed phase
- RSD:
-
Relative standard deviation
- SBSE:
-
Stir bar sorptive extraction
- SF:
-
Solvent flotation
- SLE:
-
Supported liquid extraction
- SPDE:
-
Solid phase dynamic extraction
- SPE:
-
Solid-phase extraction
- SPME:
-
Solid-phase microextraction
- SSDM:
-
Sea sand disruption method
- S-SIL:
-
Silica-supported ionic liquid
- q-TOF:
-
Time of flight
- UAE:
-
Ultrasonically assisted extraction
- UA-MSPD:
-
Ultrasound-assisted matrix solid phase dispersion
- UV:
-
Ultraviolet detection
- UPLC:
-
Ultra-performance liquid chromatography
- VA-MSPD:
-
Vortexed-assisted matrix solid phase dispersion
References
Wianowska D, Gil M, Olszowy M (2020) Miniaturized methods of sample preparation. In: Hussain Ch (ed) Handbook on miniaturization in analytical chemistry: application of nanotechnology. Elsevier, pp 99–125. https://doi.org/10.1016/b978-0-12-819763-9.00005-2
Martín-Pozo L, de Alarcón-Gómez B, Rodríguez-Gómez R, García-Córcoles MT, Çipa M, Zafra-Gómez A (2019) Analytical methods for the determination of emerging contaminants in sewage sludge samples. A review. Talanta 192:508–533. https://doi.org/10.1016/J.TALANTA.2018.09.056
Wianowska D, Gil M (2019) Recent advances in extraction and analysis procedures of natural chlorogenic acids. Phytochem Rev 18:273–302. https://doi.org/10.1007/s11101-018-9592-y
Hoff RB, Pizzolato TM (2018) Combining extraction and purification steps in sample preparation for environmental matrices: a review of matrix solid phase dispersion (MSPD) and pressurized liquid extraction (PLE) applications. TrAC—Trends Anal Chem 109:83–96. https://doi.org/10.1016/J.TRAC.2018.10.002
Wianowska D, Gil M (2019) Critical approach to PLE technique application in the analysis of secondary metabolites in plants. TrAC—Trends Anal Chem 114:314–325. https://doi.org/10.1016/j.trac.2019.03.018
Shi M-Z, Yu Y-L, Zhu S-C, Yang J, Cao J (2022) Latest development of matrix solid phase dispersion extraction and microextraction for natural products from 2015–2021. Sep Purif Rev. https://doi.org/10.1080/15422119.2022.2094274
Tu H, Chen W (2018) A review on the recent progress in matrix solid phase dispersion. Molecules 23(11):2767. https://doi.org/10.3390/molecules23112767
Capriotti AL, Cavaliere C, Foglia P, Samperi R, Stampachiacchiere S, Ventura S, Laganà A (2015) Recent advances and developments in matrix solid-phase dispersion. TrAC—Trends Anal Chem 71:186–193. https://doi.org/10.1016/j.trac.2015.03.012
Wianowska D, Gil M (2019) New insights into the application of MSPD in various fields of analytical chemistry. TrAC—Trends Anal Chem 112:29–51. https://doi.org/10.1016/j.trac.2018.12.028
El-Deen AK (2023) An overview of recent advances and applications of matrix solid-phase dispersion. Sep Purif Rev. https://doi.org/10.1080/15422119.2023.2172734
Wianowska D, Typek R, Dawidowicz AL (2015) How to eliminate the formation of chlorogenic acids artefacts during plants analysis? Sea sand disruption method (SSDM) in the HPLC analysis of chlorogenic acids and their native derivatives in plants. Phytochemistry 117:489–499. https://doi.org/10.1016/j.phytochem.2015.07.006
Wianowska D (2015) Application of sea sand disruption method for HPLC determination of quercetin in plants. J Liq Chromatogr Relat Technol 38:1037–1043. https://doi.org/10.1080/10826076.2015.1012520
Dawidowicz AL, Czapczyńska NB, Wianowska D (2013) Relevance of the sea sand disruption method (ssdm) for the biometrical differentiation of the essential-oil composition from conifers. Chem Biodivers 10:241–250. https://doi.org/10.1002/cbdv.201200001
Dawidowicz AL, Wianowska D, Rado E (2011) Matrix solid-phase dispersion with sand in chromatographic analysis of essential oils in herbs. Phytochem Anal 22:51–58. https://doi.org/10.1002/pca.1250
Sowa I, Wójciak-Kosior M, Strzemski M, Sawicki J, Staniak M, Dresler S, Szwerc W, Mołdoch J, Latalski M (2018) Silica modified with polyaniline as a potential sorbent for matrix solid phase dispersion (MSPD) and dispersive solid phase extraction (d-SPE) of plant samples. Materials 11(4):467. https://doi.org/10.3390/ma11040467
Cao Y, Tang H, Chen D, Li L (2015) A novel method based on MSPD for simultaneous determination of 16 pesticide residues in tea by LC-MS/MS. J Chromatogr B Anal Technol Biomed Life Sci 998–999:72–79. https://doi.org/10.1016/j.jchromb.2015.06.013
Medina-Dzul K, Carrera-Figueiras C, Pérez-Padilla Y, Vilchis-Nestor RA, López-Téllez G, Sánchez M, Muñoz-Rodríguez D (2015) SiO2/polyvinylimidazole hybrid polymer as a sorbent for extraction by matrix solid-phase dispersion (MSPD): synthesis, characterization, and evaluation. J Polym Res 22. https://doi.org/10.1007/s10965-015-0677-7
Hong Y, Chen L (2013) Extraction of quercetin from Herba Lysimachiae by molecularly imprinted-matrix solid phase dispersion. J Chromatogr B Anal Technol Biomed Life Sci 941:38–44. https://doi.org/10.1016/j.jchromb.2013.10.002
Yan H, Wang F, Wang H, Yang G (2012) Miniaturized molecularly imprinted matrix solid-phase dispersion coupled with high performance liquid chromatography for rapid determination of auxins in orange samples. J Chromatogr A 1256:1–8. https://doi.org/10.1016/j.chroma.2012.07.037
Chen L, Li B (2012) Determination of imidacloprid in rice by molecularly imprinted-matrix solid-phase dispersion with liquid chromatography tandem mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci 897:32–36. https://doi.org/10.1016/j.jchromb.2012.04.004
Zhou M, Hu N, Shu S, Wang M (2015) Molecularly imprinted nanomicrospheres as matrix solid-phase dispersant combined with gas chromatography for determination of four phosphorothioate pesticides in carrot and yacon. J Anal Methods Chem 2015. https://doi.org/10.1155/2015/385167
Zhang R, Xu N, Wang Y, Liu X, Wang S, Cao J (2020) Metal–organic framework assisted matrix solid-phase dispersion microextraction of saponins using response surface methodology. Electrophoresis 41(15):1354–1363. https://doi.org/10.1002/elps.202000042
Cao W, Hu SS, Ye LH, Cao J, Pang XQ, Xu JJ (2016) Trace matrix solid phase dispersion using a molecular sieve as the sorbent for the determination of flavonoids in fruit peels by ultra-performance liquid chromatography. Food Chem 190:474–480. https://doi.org/10.1016/j.foodchem.2015.05.133
Chu C, Wei M, Wang S, Zheng L, He Z, Cao J, Yan J (2017) Micro-matrix solid-phase dispersion coupled with MEEKC for quantitative analysis of lignans in Schisandrae Chinensis Fructus using molecular sieve TS-1 as a sorbent. J Chromatogr B Anal Technol Biomed Life Sci 1063:174–179. https://doi.org/10.1016/j.jchromb.2017.08.024
Wang Z, Zhang L, Li N, Lei L, Shao M, Yang X, Song Y, Yu A, Zhang H, Qiu F (2014) Ionic liquid-based matrix solid-phase dispersion coupled with homogeneous liquid-liquid microextraction of synthetic dyes in condiments. J Chromatogr A 1348:52–62. https://doi.org/10.1016/j.chroma.2014.04.086
Zhang L, Wang C, Li Z, Zhao C, Zhang H, Zhang D (2018) Extraction of acetanilides in rice using ionic liquid-based matrix solid phase dispersion-solvent flotation. Food Chem 245:1190–1195. https://doi.org/10.1016/j.foodchem.2017.11.029
Wang Z, Sun R, Wang Y, Li N, Lei L, Yang X, Yu A, Qiu F, Zhang H (2014) Determination of phenolic acids and flavonoids in raw propolis by silica-supported ionic liquid-based matrix solid phase dispersion extraction high performance liquid chromatography-diode array detection. J Chromatogr B Anal Technol Biomed Life Sci 969:205–212. https://doi.org/10.1016/j.jchromb.2014.08.022
Du K, Li J, Bai Y, An M, mei Gao X, xu Chang Y (2018) A green ionic liquid-based vortex-forced MSPD method for the simultaneous determination of 5-HMF and iridoid glycosides from Fructus Corni by ultra-high performance liquid chromatography. Food Chem 244:190–196. https://doi.org/10.1016/j.foodchem.2017.10.057
Gutiérrez-Solís MC, Muñoz-Rodríguez D, Medina-Peralta S, Carrera-Figueiras C, Ávila-Ortega A (2013) Matrix solid-phase dispersion extraction of organophosphorus pesticide using SiO2-poly(N-vinylimidazole). IOP Conf Ser Mater Sci Eng 45:5–9. https://doi.org/10.1088/1757-899X/45/1/012022
Xu JJ, Cao J, Peng LQ, Cao W, Zhu QZ, Zhang QY (2016) Characterization and determination of isomers in plants using trace matrix solid phase dispersion via ultrahigh performance liquid chromatography coupled with an ultraviolet detector and quadrupole time-of-flight tandem mass spectrometry. J Chromatogr A 1436:64–72. https://doi.org/10.1016/j.chroma.2016.01.046
León-González ME, Rosales-Conrado N (2017) Determination of ibuprofen enantiomers in breast milk using vortex-assisted matrix solid-phase dispersion and direct chiral liquid chromatography. J Chromatogr A 1514:88–94. https://doi.org/10.1016/j.chroma.2017.07.072
Cao W, Hu S-S, Ye L-H, Cao J, Xu J-J, Pang X-Q (2015) Trace-chitosan-wrapped multi-walled carbon nanotubes as a new sorbent in dispersive micro solid-phase extraction to determine phenolic compounds. J Chromatogr A 1390:13–21. https://doi.org/10.1016/j.chroma.2015.02.060
Peng LQ, Li Q, xu Chang Y, An M, Yang R, Tan Z, Hao J, Cao J, Xu J, Hu SS (2016) Determination of natural phenols in olive fruits by chitosan assisted matrix solid-phase dispersion microextraction and ultrahigh performance liquid chromatography with quadrupole time-of-flight tandem mass spectrometry. J Chromatogr A 1456:68–76. https://doi.org/10.1016/j.chroma.2016.06.011
Hertzog GI, Soares KL, Caldas SS, Primel EG (2015) Study of vortex-assisted MSPD and LC-MS/MS using alternative solid supports for pharmaceutical extraction from marketed fish. Anal Bioanal Chem 407:4793–4803. https://doi.org/10.1007/s00216-015-8685-3
Shen Q, Dong W, Yang M, Baibado JT, Wang Y, Alqouqa I, Cheung HY (2013) Lipidomic study of olive fruit and oil using TiO2 nanoparticle based matrix solid-phase dispersion and MALDI-TOF/MS. Food Res Int 54:2054–2061. https://doi.org/10.1016/j.foodres.2013.10.001
Fotouhi M, Seidi S, Shanehsaz M, Naseri MT (2017) Magnetically assisted matrix solid phase dispersion for extraction of parabens from breast milks. J Chromatogr A 1504:17–26. https://doi.org/10.1016/j.chroma.2017.05.009
Escarrone ALV, Caldas SS, Soares BM, Martins SE, Primel EG, Maia Nery LE (2014) A vortex-assisted MSPD method for triclosan extraction from fish tissues with determination by LC-MS/MS. Anal Methods 6:8306–8313. https://doi.org/10.1039/c4ay01518e
Chen JM, Yang CC, Chung WH, Ding WH (2016) Vortex-homogenized matrix solid-phase dispersion coupled with gas chromatography-electron-capture negative-ion mass spectrometry to determine halogenated phenolic compounds in seafood. RSC Adv 6:96510–96517. https://doi.org/10.1039/c6ra20680h
Chen YH, Chang CY, Ding WH (2016) Vortex-homogenized matrix solid-phase dispersion for the extraction of short chain chlorinated paraffins from indoor dust samples. J Chromatogr A 1472:129–133. https://doi.org/10.1016/j.chroma.2016.10.048
Albero B, Sánchez-Brunete C, Miguel E, Tadeo JL (2017) Application of matrix solid-phase dispersion followed by GC-MS/MS to the analysis of emerging contaminants in vegetables. Food Chem 217:660–667. https://doi.org/10.1016/j.foodchem.2016.09.017
Aznar R, Albero B, Sánchez-Brunete C, Miguel E, Martín-Girela I, Tadeo JL (2017) Simultaneous determination of multiclass emerging contaminants in aquatic plants by ultrasound-assisted matrix solid-phase dispersion and GC-MS. Environ Sci Pollut Res 24:7911–7920. https://doi.org/10.1007/s11356-016-6327-8
Barfi B, Asghari A, Rajabi M, Barfi A, Saeidi I (2013) Simplified miniaturized ultrasound-assisted matrix solid phase dispersion extraction and high performance liquid chromatographic determination of seven flavonoids in citrus fruit juice and human fluid samples: hesperetin and naringenin as biomarkers. J Chromatogr A 1311:30–40. https://doi.org/10.1016/j.chroma.2013.08.078
Zhang P, Ding J, Hou J, Zhao L, Chen Y, Ding L (2017) Dynamic microwave assisted extraction coupled with matrix solid phase dispersion for the determination of chlorfenapyr and abamectin in rice by LC-MS/MS. Microchem J 133:404–411. https://doi.org/10.1016/j.microc.2017.04.006
Wianowska D (2022) Combination of sea sand disruption method and ion-pair solid-phase extraction for effective isolation and purification of chlorogenic acid from plants prior to the HPLC determination. Molecules 27(12):5601. https://doi.org/10.3390/molecules27175601
Lomba L, Ribate P, Sangüesa E, Concha J, Garralaga P, Errazquin D, García CB, Giner B (2021) Deep eutectic solvents: are they safe? Appl Sci 11(21):10061. https://doi.org/10.3390/app112110061
Yan H, J.Qiao J, Wang H, Yang G, Row KH (2011) Molecularly imprinted solid-phase extraction combined with ultrasound-assisted dispersive liquid–liquid microextraction for the determination of four Sudan dyes in sausage samples. Analyst 136:2629–2634. https://doi.org/10.1039/c0an00951b
Deng T, Wu D, Duan C, Yan X, Du Y, Zou J, Guan Y (2017) Spatial profiling of Gibberellins in a single leaf based on microscale matrix solid-phase dispersion and precolumn derivatization coupled with ultraperformance liquid chromatography-tandem mass spectrometry. Anal Chem 89:9537–9543. https://doi.org/10.1021/acs.analchem.7b02589
Medina-Dzul K, Medina-Peralta S, Carrera-Figueiras C, Sánchez M, Muñoz-Rodríguez D (2017) Matrix solid-phase dispersion extraction of organophosphorous pesticides from beeswax. Int J Environ Anal Chem 97:831–840. https://doi.org/10.1080/03067319.2017.1363195
Wianowska D, Dawidowicz AL, Bernacik K, Typek R (2017) Determining the true content of quercetin and its derivatives in plants employing SSDM and LC-MS analysis. Eur Food Res Technol 243:27–40. https://doi.org/10.1007/s00217-016-2719-8
Typek R, Dawidowicz AL, Wianowska D, Bernacik K, Stankevič M, Gil M (2019) Formation of aqueous and alcoholic adducts of curcumin during its extraction. Food Chem 276:101–109. https://doi.org/10.1016/j.foodchem.2018.10.006
Wianowska D, Dawidowicz AL (2016) Effect of water content in extraction mixture on the pressurized liquid extraction efficiency—stability of quercetin 4′-glucoside during extraction from onions. J AOAC Int 99:744–749. https://doi.org/10.5740/jaoacint.16-0019
Teixeira DM, Patão RF, Coelho AV, da Costa CT (2006) Comparison between sample disruption methods and solid–liquid extraction (SLE) to extract phenolic compounds from Ficus carica leaves. J Chromatogr A 1103:22–28. https://doi.org/10.1016/J.CHROMA.2005.11.047
Wianowska D (2014) The influence of purge times on the yields of essential oil components extracted from plants by pressurized liquid extraction. J AOAC Int 97:1310–1316. https://doi.org/10.5740/jaoacint.13-318
Dawidowicz AL, Czapczyńska NB, Wianowska D (2012) The loss of essential oil components induced by the Purge Time in the Pressurized Liquid Extraction (PLE) procedure of Cupressus sempervirens. Talanta 94:140–145. https://doi.org/10.1016/j.talanta.2012.03.008
Gallo V, Della Posta S, Gentili A, Gherardi M, De Gara L, Fanali Ch (2023) Back-extraction applied to green matrix solid-phase dispersion for fungicides determination in tomatoes. Sep Sci Plus 2200140. https://doi.org/10.1002/sscp.202200140
Fan YB, Yin YM, Bin Jiang W, Chen YP, Yang JW, Wu J, **e MX (2014) Simultaneous determination of ten steroid hormones in animal origin food by matrix solid-phase dispersion and liquid chromatography-electrospray tandem mass spectrometry. Food Chem 142:170–177. https://doi.org/10.1016/j.foodchem.2013.06.104
Li H, Sun N, Zhang J, Liang S, Sun H (2014) Development of a matrix solid phase dispersion-high performance liquid chromatography-tandem mass spectrometric method for multiresidue analysis of 25 synthetic colorants in meat products. Anal Methods 6:537–547. https://doi.org/10.1039/c3ay41664j
Cai H, Ji S, Zhang J, Shang G, Tao G, Peng C, Chen G, Hou R, Zhang L, Wan X (2017) Determination of 11 photoinitiators and their migration into tea and milk by gas chromatography-tandem mass spectrometry (MSPD-GC-MS/MS). Anal Methods 9:2957–2963. https://doi.org/10.1039/c7ay00156h
Wianowska D, Typek R, Dawidowicz AL (2015) Chlorogenic acid stability in pressurized liquid extraction conditions. J AOAC Int 98:415–421. https://doi.org/10.5740/jaoacint.14-200
Qian ZY, Li ZG, Ma J, ting Gong J, **an QM (2017) Analysis of trace microcystins in vegetables using matrix solid-phase dispersion followed by high performance liquid chromatography triple-quadrupole mass spectrometry detection. Talanta 173:101–106. https://doi.org/10.1016/j.talanta.2017.05.079
Gao Y, Sun Y, Wang Y, Zhang J, Xu B, Zhang H, Song D (2013) A practical and rapid method for the simultaneous isolation, purification and quantification of geniposide from the fruit of Gardenia jasminoides Ellis by MSPD extraction and UFLC analysis. Anal Methods 5:4112–4118. https://doi.org/10.1039/c3ay40638e
Han Y, Wen J, Zhou T, Fan G (2015) Chemical fingerprinting of Gardenia jasminoides Ellis by HPLC-DAD-ESI-MS combined with chemometrics methods quality evaluation. Food Chem 188:648–657. https://doi.org/10.1016/j.foodchem.2015.05.039
Dawidowicz AL, Wianowska D (2009) Application of the MSPD technique for the HPLC analysis of rutin in Sambucus nigra L.: the linear correlation of the matrix solid-phase dispersion process. J Chromatogr Sci 47:914–918
Rashidipour M, Heydari R, Feizbakhsh A, Hashemi P (2015) Rapid monitoring of carvacrol in plants and herbal medicines using matrix solid-phase dispersion and gas chromatography flame ionisation detector. Nat Prod Res 29:621–627. https://doi.org/10.1080/14786419.2014.980247
Mardarowicz M, Wianowska D, Dawidowicz AL, Sawicki R (2004) Comparison of terpene composition in Engelmann Spruce (Picea engelmannii) using hydrodistillation, SPME and PLE. Zeitschrift Für Naturforsch C 59:641–648. https://doi.org/10.1515/znc-2004-9-1006
Wianowska D, Dawidowicz AL (2016) Can matrix solid phase dispersion (MSPD) be more simplified? Application of solventless MSPD sample preparation method for GC-MS and GC-FID analysis of plant essential oil components. Talanta 151:179–182. https://doi.org/10.1016/J.TALANTA.2016.01.019
Wianowska D (2014) Hydrolytical instability of hydroxyanthraquinone glycosides in pressurized liquid extraction. Anal Bioanal Chem 406:3219–3227. https://doi.org/10.1007/s00216-014-7744-5
Li M, Sun Q, Li Y, Lv M, Lin L, Wu Y, Ashfaq M, ** Yu C (2016) Simultaneous analysis of 45 pharmaceuticals and personal care products in sludge by matrix solid-phase dispersion and liquid chromatography tandem mass spectrometry. Anal Bioanal Chem 408:4953–4964. https://doi.org/10.1007/s00216-016-9590-0
Vela-Soria F, Rodríguez I, Ballesteros O, Zafra-Gómez A, Ballesteros L, Cela R, Navalón A (2014) Simplified matrix solid phase dispersion procedure for the determination of parabens and benzophenone-ultraviolet filters in human placental tissue samples. J Chromatogr A 1371:39–47. https://doi.org/10.1016/j.chroma.2014.10.063
Casado J, Castro G, Rodríguez J, Ramil M, Cela R (2015) Selective extraction of antimycotic drugs from sludge samples using matrix solid-phase dispersion followed by on-line clean-up. Anal Bioanal Chem 407:907–917. https://doi.org/10.1007/s00216-014-8167-z
Guerra E, Celeiro M, Lamas JP, Llompart M, Garcia-Jares C (2015) Determination of dyes in cosmetic products by micro-matrix solid phase dispersion and liquid chromatography coupled to tandem mass spectrometry. J Chromatogr A 1415:27–37. https://doi.org/10.1016/j.chroma.2015.08.054
Li J, Li Y, Xu D, Zhang J, Wang Y, Luo C (2017) Determination of metrafenone in vegetables by matrix solid-phase dispersion and HPLC-UV method. Food Chem 214:77–81. https://doi.org/10.1016/j.foodchem.2016.07.061
Kaur P, Kaur K, Bhullar MS (2014) Quantification of penoxsulam in soil and rice samples by matrix solid phase extraction and liquid-liquid extraction followed by HPLC-UV method. Environ Monit Assess 186:7555–7563. https://doi.org/10.1007/s10661-014-3947-7
Villaverde-de-Sáa E, Rodil R, Quintana JB, Cela R (2016) Matrix solid-phase dispersion combined to liquid chromatography–tandem mass spectrometry for the determination of paraben preservatives in mollusks. J Chromatogr A 1459:57–66. https://doi.org/10.1016/j.chroma.2016.06.070
Iparraguirre A, Rodil R, Quintana JB, Bizkarguenaga E, Prieto A, Zuloaga O, Cela R, Fernández LA (2014) Matrix solid-phase dispersion of polybrominated diphenyl ethers and their hydroxylated and methoxylated analogues in lettuce, carrot and soil. J Chromatogr A 1360:57–65. https://doi.org/10.1016/j.chroma.2014.07.079
Samanidou VF, Frysali MA, Papadoyannis IN (2014) Matrix solid phase dispersion for the extraction of bisphenol a from human breast milk prior to HPLC analysis. J Liq Chromatogr Relat Technol 37:247–258. https://doi.org/10.1080/10826076.2012.745133
Kamal YT, Alam P, Alqasoumi SI, Foudah AI, Alqarni MH, Yusufoglu HS (2018) Investigation of antioxidant compounds in commercial pomegranate molasses products using matrix-solid phase dispersion extraction coupled with HPLC. Saudi Pharm J 26:839–844. https://doi.org/10.1016/j.jsps.2018.03.015
Argente-García A, Moliner-Martínez Y, Campíns-Falcó P, Verdú-Andrés J, Herráez-Hernández J (2016) Determination of amphetamines in hair by integrating sample disruption, clean-up and solid phase derivatization. J Chromatogr A 1447:47–56. https://doi.org/10.1016/j.chroma.2016.04.036
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
The authors have declared no conflict of interest.
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Wianowska, D., Olszowy-Tomczyk, M. (2024). Matrix Solid-Phase Disperion. In: Rodríguez-Delgado, M.Á., Socas-Rodríguez, B., Herrera-Herrera, A.V. (eds) Microextraction Techniques. Integrated Analytical Systems. Springer, Cham. https://doi.org/10.1007/978-3-031-50527-0_6
Download citation
DOI: https://doi.org/10.1007/978-3-031-50527-0_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-50526-3
Online ISBN: 978-3-031-50527-0
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)