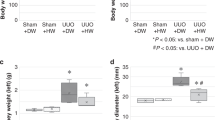
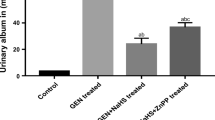
Abstract
In prolonged complete unilateral ureteral obstruction, reduced renal blood flow places the kidney in a state of ischemia, which can cause tubular injury and inflammation. Infiltrating inflammatory cells release transforming growth factor-beta 1 (TGF-β1), a cytokine that initiates fibrosis through epithelial-mesenchymal transition (EMT) pathway. Persistent fibrosis can lead to irreversible renal injury and loss of renal function. While surgical intervention can remove the obstruction, relief of obstruction may not fully reverse renal injury. Additionally, patients often encounter long wait times between initial consultation and medical intervention, resulting in the accumulation of renal injury that may cause permanent dysfunction. Currently, accepted pharmacological therapies to mitigate the symptoms of obstructive nephropathy include acetaminophen, cyclooxygenase inhibitors, nonsteroidal anti-inflammatory medications, opioids, and alpha-receptor blockers. However, there is no evidence that they mitigate renal injury. Therefore, identifying potential therapies that could be administered during obstruction may help to improve renal function following decompression. Scientific evidence suggests that endogenously produced gasotransmitters can exhibit anti-inflammatory and antioxidant effects. Nitric oxide, carbon monoxide, and hydrogen sulfide have been identified as gasotransmitters and have been shown to have cytoprotective effects in various models of tissue injury. Studies have shown that treatment with sodium hydrogen sulfide (a hydrogen sulfide donor salt) mitigated TGF-β1 expression, oxidative stress, fibrosis, and inflammation associated with urinary obstruction. More recently, the use of more directed hydrogen sulfide donor molecules, such as GYY4137, has led to significant decreases in inflammation, fibrosis, and expression of EMT markers following urinary obstruction. Taken together, these findings suggest that hydrogen sulfide may be a novel potential therapy against obstructive nephropathy. This chapter focuses on the pathogenesis and treatment of obstructive nephropathy and proposes novel upcoming strategies that could improve patient outcomes.
This chapter is a modified version by the same authors in the publication titled Is Hydrogen Sulfide a Potential Novel Therapy to Prevent Renal Damage During Ureteral Obstruction? Nitric Oxide. 2018; 73:15–21.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Roth KS, Koo HP, Spottswood SE, Chan JCM. Obstructive uropathy: an important cause chronic renal failure in children. Clin Pediatr (Phila). 2002;41:309–14.
Spernat D, Kourambas J. Urolithiasis—medical therapies. BJU Int. 2011;108:9–13.
Klahr S. Obstructive nephropathy. Intern Med. 2000;39(5):355–61.
Ucero AC, Benito-Martin A, Izquierdo MC, Sanchez-Niño MD, Sanz AB, Ramos AM, et al. Unilateral ureteral obstruction: beyond obstruction. Int Urol Nephrol. 2014;46(4):765–76.
Truong LD, Choi J-J, Tsao CC, Ayala G, Sheikh-Hamad D, Nassar G, et al. Renal cell apoptosis in chronic obstructive uropathy: the roles of caspases. Kidney Int. 2001;60:924–34.
Lech M, Anders H-J. Macrophages and fibrosis: how resident and infiltrating mononuclear phagocytes orchestrate all phases of tissue injury and repair. Biochim Biophys Acta. 2013;1832(7):989–97.
Kluth DC, Erwig L-P, Rees AJ. Multiple facets of macrophages in renal injury. Kidney Int. 2004;66:542–57.
Sakurai H, Hisada Y, Ueno M, Sugiura M, Kawashima K, Sugita T. Activation of transcription factor NF-kB in experimental glomerulonephritis in rats. Biochim Biophys Acta. 1996;1316:132–8.
Eddy AA, López-Guisa JM, Okamura DM, Yamaguchi I. Investigating mechanisms of chronic kidney disease in mouse models. Pediatr Nephrol. 2012;27(8):1233–47.
Samarakoon R, Overstreet JM, Higgins SP, Higgins PJ. TGF-β1 → SMAD/p53/USF2 → PAI-1 transcriptional axis in ureteral obstruction-induced renal fibrosis. Cell Tissue Res. 2012;347(1):117–28.
Chevalier RL. Pathogenesis of renal injury in obstructive uropathy. Curr Opin Pediatr. 2006;18(2):153–60.
Iwano M, Neilson EG. Mechanisms of tubulointerstitial fibrosis. Curr Opin Nephrol Hypertens. 2004;13:279–84.
Kaneto H, Morrissey J, Klahr S. Increased expression of TGF-β1 mRNA in the obstructed kidney of rats with unilateral ureteral ligation. Kidney Int. 1993;44(2):313–21.
Manucha W, Oliverros L, Carrizo L, Seltzer A, Valles P. Losartan modulation on NOS isoforms and COX-2 expression in early renal fibrogenesis in unilateral obstruction. Kidney Int. 2004;65:2091–107.
Gibbons GH, Pratt RE, Dzau VJ. Vascular smooth muscle cell hypertrophy vs. hyperplasia autocrine transforming growth factor beta 1, expression determines growth response to angiotensin II. J Clin Invest. 1992;90:456–61.
Xu J, Lamouille S, Derynck R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009;19(2):156–72.
Vaughan ED Jr, Gillenwater JY. Recovery following complete chronic unilateral ureteral occlusion: functional, radiographic and pathologic alterations. J Urol. 1971;106:27–35.
Chevalier RL, Kim A, Thornhill BA, Wolstenholme JT. Recovery following relief of unilateral ureteral obstruction in the neonatal rat. Kidney Int. 1999;55:793–807.
Chaabane W, Praddaude F, Buleon M, Jaafar A, Vallet M, Rischmann P, et al. Renal functional decline and glomerulotubular injury are arrested but not restored by release of unilateral ureteral obstruction (UUO). Am J Physiol Ren Physiol. 2013;304(4):F432–9.
Chevalier RL, Forbes MS, Thornhill BA. Ureteral obstruction as a model of renal interstitial fibrosis and obstructive nephropathy. Kidney Int. 2009;75(11):1145–52.
Tapmeier TT, Brown KL, Tang Z, Sacks SH, Sheerin NS, Wong W. Reimplantation of the ureter after unilateral ureteral obstruction provides a model that allows functional evaluation. Kidney Int. 2008;73(7):885–9.
York NE, Borofsky MS, Lingeman JE. Risks associated with drug treatments for kidney stones. Expert Opin Drug Saf. 2015;14:1865–77.
Kerr WS Jr. Effects of complete ureteral obstruction in dogs on kidney function. Am J Phys. 1956;184:521–6.
Wu AK, Tran TC, Sorensen MD, Durack JC, Stoller ML. Relative renal function does not improve after relieving chronic renal obstruction. BJU Int. 2012;109(10):1540–4.
Wang R. Two’s company, three’s a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J. 2002;16(13):1792–8.
Qian Y, Matson JB. Gasotransmitter delivery via self-assembling peptides: treating diseases with natural signaling gases. Adv Drug Deliv Rev. 2017;110:137–56.
Sharma JN, Al-Omran A, Parvathy SS. Role of nitric oxide in inflammatory diseases. Inflammopharmacology. 2007;15(6):252–9.
Lefer AM, Lefer DJ. Nitric oxide. II. Nitric oxide protects in intestinal inflammation. Am J Phys. 1999;276(3 Pt 1):G572–5.
Sun D, Wang Y, Liu C, Zhou X, Li X, **ao A. Effects of nitric oxide on renal interstitial fibrosis in rats with unilateral ureteral obstruction. Life Sci. 2012;90(23–24):900–9.
Gibbons SJ, Farrugia G. The role of carbon monoxide in the gastrointestinal tract. J Physiol. 2004;556(Pt 2):325–36.
Motterlini R, Foresti R. Heme oxygenase-1 as a target for drug discovery. Antioxid Redox Signal. 2013;20(11):1810–26.
Abe T, Fu**o M, Yazawa K, Imamura R, Hatayama N, Kakuta Y, Tsutahara K, Okumi M, Ichimaru N, Kaimori JY, Isaka Y, Seki K, Takahara S, Li X-K, Nonomura N. High-pressure carbon monoxide preserves rat kidney grafts from apoptosis and inflammation. Lab Investig. 2017;97:468–77.
Bauer I, Pannen BHJ. Bench-to-bedside review: carbon monoxide—from mitochondrial poisoning to therapeutic use. Crit Care. 2009;13(4):1–10.
Wallace JL, Ianaro A, Flannigan KL, Cirino G. Gaseous mediators in resolution of inflammation. Semin Immunol. 2015;27(3):227–33.
Whiteman M, Le Trionnaire S, Chopra M, Fox B, Whatmore J. Emerging role of hydrogen sulfide in health and disease: critical appraisal of biomarkers and pharmacological tools. Clin Sci. 2011;121(11):459–88.
Lobb I, Sonke E, Aboalsamh G, Sener A. Hydrogen sulphide and the kidney: important roles in renal physiology and pathogenesis and treatment of kidney injury and disease. Nitric Oxide. 2014;46(2015):55–65.
Yamamoto J, Sato W, Kosugi T, Yamamoto T, Kimura T, Taniguchi S, et al. Distribution of hydrogen sulfide (H2S)-producing enzymes and the roles of the H2S donor sodium hydrosulfide in diabetic nephropathy. Clin Exp Nephrol. 2013;17(1):32–40.
**a M, Chen L, Muh RW, Li P, Li N. Production and actions of hydrogen sulfide, a novel gaseous bioactive substance, in the kidneys. J Pharmacol Exp Ther. 2006;329(3):1056–62.
Xue H, Yuan P, Ni J, Li C, Shao D, Liu J, et al. H2S inhibits hyperglycemia-induced intrarenal renin–angiotensin system activation via attenuation of reactive oxygen species generation. PLoS One. 2013;8(9):e74366.
Jung K-J, Jang H-S, Kim JI, Han SJ, Park J-W, Park KM. Involvement of hydrogen sulfide and homocysteine transsulfuration pathway in the progression of kidney fibrosis after ureteral obstruction. Biochim Biophys Acta. 2013;1832(12):1989–97.
Song K, Li Q, Yin X-Y, Lu Y, Liu C-F, Hu L-F. Hydrogen sulfide: a therapeutic candidate for fibrotic disease? Oxid Med Cell Longev. 2015;2015:458720.
Whiteman M, Armstrong JS, Chu SH, Jia-Ling S, Wong BS, Cheung NS, et al. The novel neuromodulator hydrogen sulfide: an endogenous peroxynitrite “scavenger”? J Neurochem. 2004;90(3):765–8.
Deng Y. Protective effects of hydrogen sulfide on oxidative stress and fibrosis in hepatic stellate cells. Mol Med Rep. 2012;7:247–53.
Jiang D, Zhang Y, Yang M, Wang S, Jiang Z, Li Z. Exogenous hydrogen sulfide prevents kidney damage following unilateral ureteral obstruction. Neurourol Urodyn. 2014;33:538–43.
Cao H, Zhou X, Zhang J, Huang X, Zhai Y, Zhang X, et al. Hydrogen sulfide protects against bleomycin-induced pulmonary fibrosis in rats by inhibiting NF-κB expression and regulating Th1/Th2 balance. Toxicol Lett. 2014;224(3):387–94.
Szabó C. Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discov. 2007;6(11):917–35.
Lobb I, Mok A, Lan Z, Liu W, Garcia B, Sener A. Supplemental hydrogen sulphide protects transplant kidney function and prolongs recipient survival after prolonged cold ischemia-reperfusion injury by mitigating renal graft apoptosis and inflammation. BJU Int. 2012;110(11 Pt C):E1187–95.
Dursun M, Alper O, Emin O, Suleyman S, Huseyin B, Ozgur DO, Cekmen Mustafa NO, Somay A. Protective effect of hydrogen sulfide on protective effect of hydrogen sulfide on renal injury in the experimental unilateral ureteral obstruction-induced renal injury. Int Braz J Urol. 2015;41:1185–93.
Song K, Wang F, Li Q, Shi Y-B, Zheng H-F, Peng H, et al. Hydrogen sulfide inhibits the renal fibrosis of obstructive nephropathy. Kidney Int. 2014;85(6):1318–29.
Meng X-M, Nikolic-Paterson DJ, Lan HY. Inflammatory processes in renal fibrosis. Nat Rev Nephrol. 2014;10(9):493–503.
Zhang S, Pan C, Zhou F, Yuan Z, Wang H, Cui W, et al. Hydrogen sulfide as a potential therapeutic target in fibrosis. Oxid Med Cell Longev. 2015;2015:593407.
Fang L-P, Lin Q, Tang C-S, Liu X-M. Hydrogen sulfide attenuates epithelial-mesenchymal transition of human alveolar epithelial cells. Pharmacol Res. 2010;61(4):298–305.
Zheng D, Dong S, Li T, Yang F, Yu X, Wu J, et al. Exogenous hydrogen sulfide attenuates cardiac fibrosis through reactive oxygen species signal pathways in experimental diabetes mellitus models. Cell Physiol Biochem. 2015;36(3):917–29.
Lin S, Visram F, Liu W, Haig A, Jiang J, Mok A, et al. GYY4137, a slow-releasing hydrogen sulfide donor, ameliorates renal damage associated with chronic obstructive uropathy. J Urol. 2016;196:1778–87.
Guo L, Peng W, Tao J, Lan Z, Hei H, Tian L, et al. Hydrogen sulfide inhibits transforming growth factor-β1-induced EMT via Wnt/catenin pathway. PLoS One. 2016;11(1):e01470181.
Li L, Whiteman M, Guan YY, Neo KL, Cheng Y, Lee SW, et al. Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): new insights into the biology of hydrogen sulfide. Circulation. 2008;117(18):2351–60.
Li L, Fox B, Keeble J, Salto-Tellez M, Winyard PG, Wood ME, et al. The complex effects of the slow-releasing hydrogen sulfide donor GYY4137 in a model of acute joint inflammation and in human cartilage cells. J Cell Mol Med. 2013;17(3):365–76.
Wallace JL, Vaughan D, Dicay M, MacNaughton WK, DeNucci G. Hydrogen sulfide-releasing therapeutics: translation to the clinic. Antioxid Redox Signal. 2018;28(16):1533–40.
Szabo C, Papapetropoulos A. International union of basic and clinical pharmacology. CII: pharmacological modulation of H2S levels: H2S donors and H2S biosynthesis inhibitors. Pharmacol Rev. 2017;69(4):497–564.
Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO J. 2001;20(21):6008–16.
Altaany Z, Yang G, Wang R. Crosstalk between hydrogen sulfide and nitric oxide in endothelial cells. J Cell Mol Med. 2013;17(7):879–88.
Hanna N, Cardin S, Leung TK, Nattel S. Differences in atrial versus ventricular remodeling in dogs with ventricular tachypacing-induced congestive heart failure. Cardiovasc Res. 2004;63(2):236–44.
Kondo K, Bhushan S, King A, Prabhu S, Hamid T, Koenig S, et al. H2S protects against pressure overload induced heart failure via upregulation of endothelial nitric oxide synthase (eNOS). Circulation. 2014;127(10):1116–27.
Feliers D, Lee HJHJ, Kasinath BSBS. Hydrogen sulfide in renal physiology and disease. Antioxid Redox Signal. 2016;25(13):720–31.
Zhou X, Feng Y, Zhan Z, Chen J. Hydrogen sulfide alleviates diabetic nephropathy in a streptozotocin-induced diabetic rat model. J Biol Chem. 2014;289(42):28827–34.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
None.
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Lin, S., Juriasingani, S., Dugbartey, G.J., Sener, A. (2023). Hydrogen Sulfide for Prevention of Obstructive Nephropathy. In: Hydrogen Sulfide in Kidney Diseases. Springer, Cham. https://doi.org/10.1007/978-3-031-44041-0_7
Download citation
DOI: https://doi.org/10.1007/978-3-031-44041-0_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-44040-3
Online ISBN: 978-3-031-44041-0
eBook Packages: MedicineMedicine (R0)