Abstract
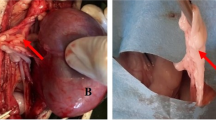
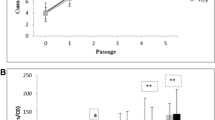
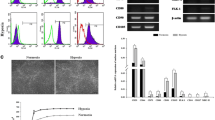
Canine adipose-derived mesenchymal stem cells (cAD-MSCs) have been demonstrated to be effective in treating several illnesses due to, among others, their differentiation (DI) potential. However, some treatments require in vitro multiplication of cAD-MSCs, which may alter their DI characteristics. This research aimed to determine if in vitro cultivation affects DI processes in the molecular profiles of cAD-MSCs. Isolation of cAD-MSCs from the abdominal adipose tissue of eight young female dogs was performed, and their immunophenotype and in vitro DI potential was confirmed. Total RNA was extracted in the early passage (P) 3 and late P6, and changes in the gene expression profile linked to DI were examined using a microarray. Isolated cAD-MSCs arrested proliferation at P8, on average. They displayed typical immunophenotypes and maintained their DI potential during in vitro cultivation. Gene up- and downregulations were observed; however, the overall expression was not significantly altered comparing P3 to P6. The obtained results of up- and downregulations suggest in vitro cultivation may favour DI events, as indicated by the overexpression of the Growth DI factor 7 (GDF7), a crucial gene for cartilage formation, Kinase insert domain receptor (KDR), a protein involved in angiogenesis during DI, and Fibroblast growth factor 10 (FGF10), a protein required for the development of embryonic limbs. However, T-box transcription factor (TBX5), a promoter of osteoDI and mineralization, was downregulated in P6, suggesting prolonged in vitro cultivation could influence the osteoDI potential. The downregulation of Ras Homolog Family Member (RHOA) could be advantageous since overexpression of this gene is linked to the proliferation and spread of malignant cells. More donors and a comparison of the genders should be included in future research. Thus, this research demonstrated that, despite minor alterations, in vitro multiplication did not significantly alter the DI potential and gene expression of young female cAD-MSCs, which is a vital prerequisite for producing high-quality canine stem cell products.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Qu-Petersen, Z., et al.: Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. J. Cell Biol. 157, 851–864 (2002). https://doi.org/10.1083/jcb.200108150
Erices, A., Conget, P., Minguell, J.J.: Mesenchymal progenitor cells in the human umbilical cord. Br. J. Haematol. 109, 235–242 (2000). https://doi.org/10.1007/s00277-004-0918-z
Gronthos, S., Mankani, M., Brahim, J., Robey, P.G., Shi, S.: Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. U. S. A. 97, 13625–13630 (2000). https://doi.org/10.1073/pnas.240309797
Miao, Z., et al.: Isolation of mesenchymal stem cells from human placenta: comparison with human bone marrow mesenchymal stem cells. Cell Biol. Int. 30, 681–687 (2006). https://doi.org/10.1016/j.cellbi.2006.03.009
Alviano, F., et al.: Term amniotic membrane is a high throughput source for multipotent mesenchymal stem cells with the ability to differentiate into endothelial cells in vitro. BMC Dev. Biol. 7, 1–14 (2007). https://doi.org/10.1186/1471-213X-7-11
Friedenstein, A.J., Petrakova, K.V., Kurolesova, A.I., Frolova, G.P.: Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation 6, 230–247 (1968)
Zuk, P.A., et al.: Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 7, 211–228 (2001). https://doi.org/10.1089/107632701300062859
Neupane, M., Chang, C.C., Kiupel, M., Yuzbasiyan-Gurkan, V.: Isolation and characterization of canine adipose-derived mesenchymal stem cells. Tissue Eng. - Part A. 14, 1007–1015 (2008). https://doi.org/10.1089/ten.tea.2007.0207
Dabrowska, S., Andrzejewska, A., Janowski, M., Lukomska, B.: Immunomodulatory and regenerative effects of mesenchymal stem cells and extracellular vesicles: therapeutic outlook for inflammatory and degenerative diseases. Front. Immunol. 11, 591065 (2021). https://doi.org/10.3389/fimmu.2020.591065
Vieira, N.M., Brandalise, V., Zucconi, E., Secco, M., Strauss, B.E., Zatz, M.: Isolation, characterization, and differentiation potential of canine adipose-derived stem cells. Cell Transplant. 19, 279–289 (2010). https://doi.org/10.3727/096368909X481764
Blecker, D., Elashry, M.I., Heimann, M., Wenisch, S., Arnhold, S.: New insights into the neural differentiation potential of canine adipose tissue-derived mesenchymal stem cells. J. Vet. Med. Ser. C Anat. Histol. Embryol. 46, 304–315 (2017). https://doi.org/10.1111/ahe.12270
Prpar Mihevc, S., Kokondoska Grgich, V., Kopitar, A.N., Mohorič, L., Majdič, G.: Neural differentiation of canine mesenchymal stem cells/multipotent mesenchymal stromal cells. BMC Vet. Res. 16, 1–12, 282 (2020). https://doi.org/10.1186/s12917-020-02493-2
Shah, K., et al.: Outcome of allogeneic adult stem cell therapy in dogs suffering from osteoarthritis and other joint defects. Stem Cells Int. 2018, 7309201 (2018). https://doi.org/10.1155/2018/7309201
Kaur, G., et al.: A double-blinded placebo-controlled evaluation of adipose-derived mesenchymal stem cells in treatment of canine atopic dermatitis. Vet. Res. Commun. 46(1), 251–260 (2021). https://doi.org/10.1007/s11259-021-09853-9
Falcão, M.S.A., et al.: Effect of allogeneic mesenchymal stem cells (MSCs) on corneal wound healing in dogs. J. Tradit. Complement. Med. 10, 440–445 (2020). https://doi.org/10.1016/j.jtcme.2019.04.006
Bach, F.S., et al.: Comparison of the efficacy of surgical decompression alone and combined with canine adipose tissue-derived stem cell transplantation in dogs with acute thoracolumbar disk disease and spinal cord injury. Front. Vet. Sci. 6, 1–11, 383 (2019). https://doi.org/10.3389/fvets.2019.00383
Pérez-Merino, E.M., et al.: Safety and efficacy of allogeneic adipose tissue-derived mesenchymal stem cells for treatment of dogs with inflammatory bowel disease: clinical and laboratory outcomes. Vet. J. 206, 385–390 (2015). https://doi.org/10.1016/j.tvjl.2015.08.003
Yan, Y., et al.: Therapeutic applications of adipose-derived mesenchymal stem cells on acute liver injury in canines. Res. Vet. Sci. 126, 233–239 (2019). https://doi.org/10.1016/j.rvsc.2019.09.004
Lee, K.S., et al.: Sequential sub-passage decreases the differentiation potential of canine adipose-derived mesenchymal stem cells. Res. Vet. Sci. 96, 267–275 (2014). https://doi.org/10.1016/j.rvsc.2013.12.011
Requicha, J.F., Viegas, C.A., Albuquerque, C.M., Azevedo, J.M., Reis, R.L., Gomes, M.E.: Effect of anatomical origin and cell passage number on the stemness and osteogenic differentiation potential of canine adipose-derived stem cells. Stem Cell Rev. Reports. 8, 1211–1222 (2012). https://doi.org/10.1007/s12015-012-9397-0
Screven, R., et al.: Immunophenotype and gene expression profile of mesenchymal stem cells derived from canine adipose tissue and bone marrow. Vet. Immunol. Immunopathol. 161, 21–31 (2014). https://doi.org/10.1016/j.vetimm.2014.06.002
Lee, J., et al.: Effect of donor age on the proliferation and multipotency of canine adipose-derived mesenchymal stem cells. J. Vet. Sci. 18, 141–148 (2017). https://doi.org/10.4142/jvs.2017.18.2.141
Zhao, Y., Waldman, S.D., Flynn, L.E.: The effect of serial passaging on the proliferation and differentiation of bovine adipose-derived stem cells. Cells Tissues Organs 195, 414–427 (2012). https://doi.org/10.1159/000329254
Yang, Y.H.K., Ogando, C.R., Wang See, C., Chang, T.Y., Barabino, G.A.: Changes in phenotype and differentiation potential of human mesenchymal stem cells aging in vitro. Stem Cell Res. Ther. 9, 1–14, 131 (2018). https://doi.org/10.1186/s13287-018-0876-3
Krešić, N., et al.: The expression pattern of surface markers in canine adipose‐derived mesenchymal stem cells. Int. J. Mol. Sci. 22, 7476 (2021). https://doi.org/10.3390/ijms22147476
Krešić, N., Šimić, I., Lojkić, I., Tomislav, B.: Canine adipose derived mesenchymal stem cells transcriptome composition alterations : a step towards standardizing therapeutic. Stem Cells Int. 2017, 4176292 (2017). https://doi.org/10.1155/2017/4176292
Dominici, M., et al.: Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 8, 315–317 (2006). https://doi.org/10.1080/14653240600855905
Selle, M., et al.: Influence of age on stem cells depends on the sex of the bone marrow donor. J. Cell. Mol. Med. 26, 1594–1605 (2022). https://doi.org/10.1111/jcmm.17201
Martinello, T., et al.: Canine adipose-derived-mesenchymal stem cells do not lose stem features after a long-term cryopreservation. Res. Vet. Sci. 91, 18–24 (2011). https://doi.org/10.1016/j.rvsc.2010.07.024
Guercio, A., Di Bella, S., Casella, S., Marco, P.D., Russo, C., Piccione, G.: Canine mesenchymal stem cells (MSCs): characterization in relation to donor age and adipose tissue-harvesting site. Cell Biol. Int. 37, 789–798 (2013). https://doi.org/10.1002/cbin.10090
Sasaki, A., et al.: Canine mesenchymal stem cells from synovium have a higher chondrogenic potential than those from infrapatellar fat pad, adipose tissue, and bone marrow. PLoS ONE 13, 1–20, e0202922 (2018). https://doi.org/10.1371/journal.pone.0202922
Rashid, U., et al.: Characterization and differentiation potential of mesenchymal stem cells isolated from multiple canine adipose tissue sources. BMC Vet. Res. 17, 1–12, 388 (2021). https://doi.org/10.1186/s12917-021-03100-8
Voga, M., Kovač, V., Majdic, G.: Comparison of canine and feline adipose-derived mesenchymal stem cells/medicinal signaling cells with regard to cell surface marker expression, viability, proliferation, and differentiation potential. Front. Vet. Sci. 7, 1–13, 610240 (2021). https://doi.org/10.3389/fvets.2020.610240
Tan, K., et al.: CD73 expression on mesenchymal stem cells dictates the reparative properties via its anti-inflammatory activity. Stem Cells Int. 2019, 8717694 (2019). https://doi.org/10.1155/2019/8717694
Takemitsu, H., Zhao, D., Yamamoto, I., Harada, Y., Michishita, M., Arai, T.: Comparison of bone marrow and adipose tissue-derived canine mesenchymal stem cells. BMC Vet. Res. 8, 150 (2012). https://doi.org/10.1186/1746-6148-8-150
Davies, O.G., Cooper, P.R., Shelton, R.M., Smith, A.J., Scheven, B.A.: Isolation of adipose and bone marrow mesenchymal stem cells using CD29 and CD90 modifies their capacity for osteogenic and adipogenic differentiation. J. Tissue Eng. 6, (2015). https://doi.org/10.1177/2041731415592356
James, J.L., et al.: The chondrogenic potential of first-trimester and term placental mesenchymal stem/stromal cells. Cartilage. 13, 544–558 (2021). https://doi.org/10.1177/19476035211044822
Mikic, B., Ferreira, M.P., Battaglia, T.C., Hunziker, E.B.: Accelerated hypertrophic chondrocyte kinetics in GDF-7 deficient murine tibial growth plates. J. Orthop. Res. 26, 986–990 (2008). https://doi.org/10.1002/jor.20574
Kumlin, M., Lindberg, K., Haldosen, L.-A., Felländer-Tsai, L., Li, Y.: Growth differentiation factor 7 promotes multiple-lineage differentiation in tenogenic cultures of mesenchymal stem cells. Injury 53, 4165–4168 (2022). https://doi.org/10.1016/J.INJURY.2022.09.017
Tsuji-Tamura, K., Ogawa, M.: Morphology regulation in vascular endothelial cells. Inflamm. Regen. 38, 1–13, 25 (2018). https://doi.org/10.1186/s41232-018-0083-8
Xu, X., Weinstein, M., Li, C., Deng, C.X.: Fibroblast growth factor receptors (FGFRs) and their roles in limb development. Cell Tissue Res. 296, 33–43 (1999). https://doi.org/10.1007/s004410051264
Wang, J., et al.: Mechanical stimulation orchestrates the osteogenic differentiation of human bone marrow stromal cells by regulating HDAC1. Cell Death Dis. 7, 1–12 (2016). https://doi.org/10.1038/cddis.2016.112
Lee, H.W., Suh, J.H., Kim, A.Y., Lee, Y.S., Park, S.Y., Kim, J.B.: Histone deacetylase 1-mediated histone modification regulates osteoblast differentiation. Mol. Endocrinol. 20, 2432–2443 (2006). https://doi.org/10.1210/me.2006-0061
Li, J., et al.: TRAF4 positively regulates the osteogenic differentiation of mesenchymal stem cells by acting as an E3 ubiquitin ligase to degrade Smurf2. Cell Death Differ. 26, 2652–2666 (2019). https://doi.org/10.1038/s41418-019-0328-3
Kolf, C.M., Cho, E., Tuan, R.S.: Mesenchymal stromal cells. Biology of adult mesenchymal stem cells: regulation of niche, self-renewal and differentiation. Arthritis Res. Ther. 9, 1–10, 204 (2007). https://doi.org/10.1186/ar2116
Fink, T., et al.: Induction of adipocyte-like phenotype in human mesenchymal stem cells by hypoxia. Stem Cells. 22, 1346–1355 (2004). https://doi.org/10.1634/stemcells.2004-0038
Haga, R.B., Ridley, A.J.: Rho GTPases: regulation and roles in cancer cell biology. Small GTPases. 7, 207–221 (2016). https://doi.org/10.1080/21541248.2016.1232583
Acknowledgments
We thank Mihaela Stuparić Komušar for her generous help during spheroid preparation and staining.
Funding
This research was funded by Croatian Science Foundation (HRZZ) and performed within Installation Research Project (UIP-2019–04-2178) “Revealing transcriptome and secretome of mesenchymal stem cells” SECRET. We thank HRZZ for supporting our research.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Prišlin, M. et al. (2024). Influence of In Vitro Cultivation on Differentiation Gene Expressions in Canine Adipose-Derived Mesenchymal Stem Cells. In: Bonačić Bartolin, P., Magjarević, R., Allen, M., Sutcliffe, M. (eds) Advances in Biomedical and Veterinary Engineering. BioMedVetMech 2022. IFMBE Proceedings, vol 90. Springer, Cham. https://doi.org/10.1007/978-3-031-42243-0_1
Download citation
DOI: https://doi.org/10.1007/978-3-031-42243-0_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-42242-3
Online ISBN: 978-3-031-42243-0
eBook Packages: EngineeringEngineering (R0)